Preparation method of diclofenac sodium sustained release preparation
A technology of diclofenac sodium and sustained-release preparations, applied in the directions of anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve problems such as changes in drug release, unfavorable oral administration, and large dosage of excipients, and achieve stable release and sustained-release functions. Good and productive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
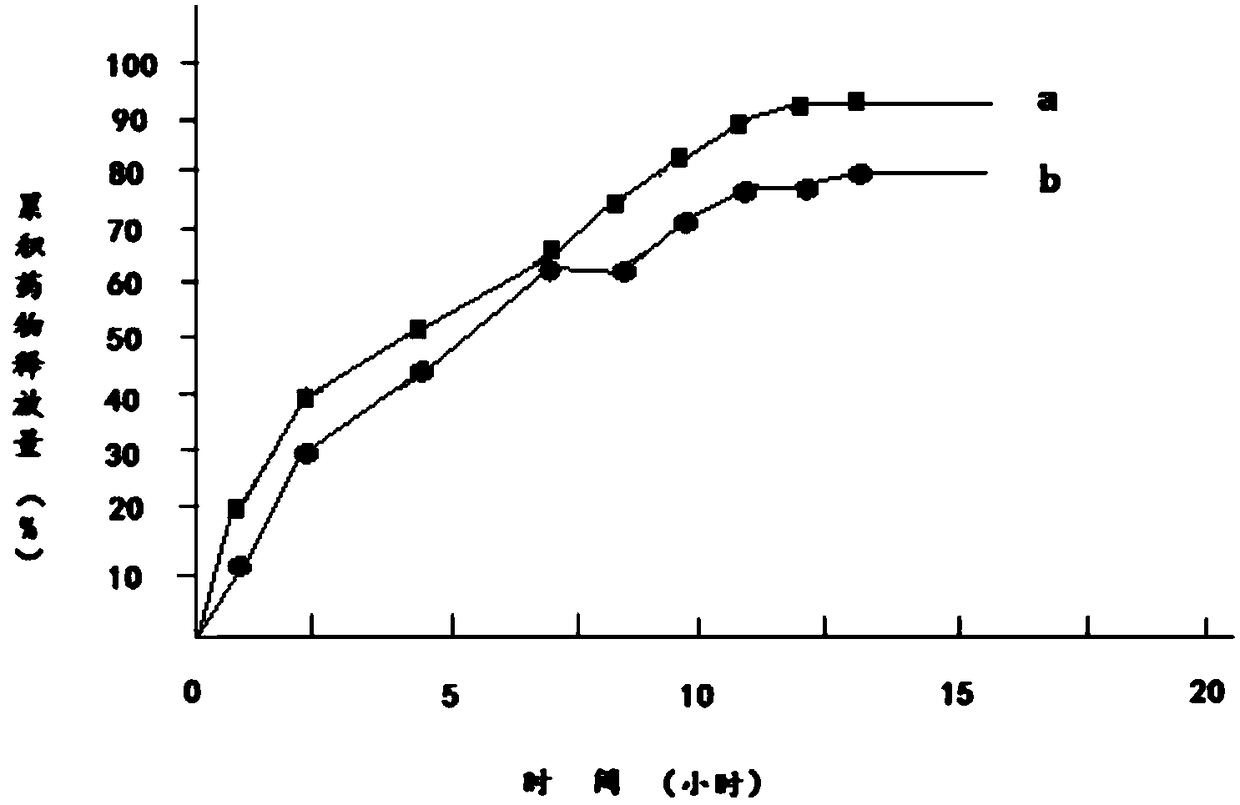
Embodiment 1
[0023] 1) Dissolve 100g of diclofenac sodium in 100g of 95% ethanol to obtain diclofenac sodium ethanol solution, which is referred to as solution A;
[0024] 2) Add 150g of hydroxypropyl β-cyclodextrin and 20g of mannitol to solution A to obtain solution B;
[0025] 3) Dissolve 100g of polylactic acid and 20g of polyethylene glycol 200 in 180g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is referred to as solution C;
[0026] 4) Mix solution B and solution C to obtain solution D;
[0027] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15°C to 18°C, continuously stir solution D for 12 hours, then reduce the temperature of solution D to 0°C to 1°C within 2 hours and let it stand for 12 hours. Hours, keep the temperature of solution D at 0°C to 1°C during the standing period;
[0028] 6) Heat the solution D obtained in step 5, and stir continuously for 12 hours when the temperature of the solution...
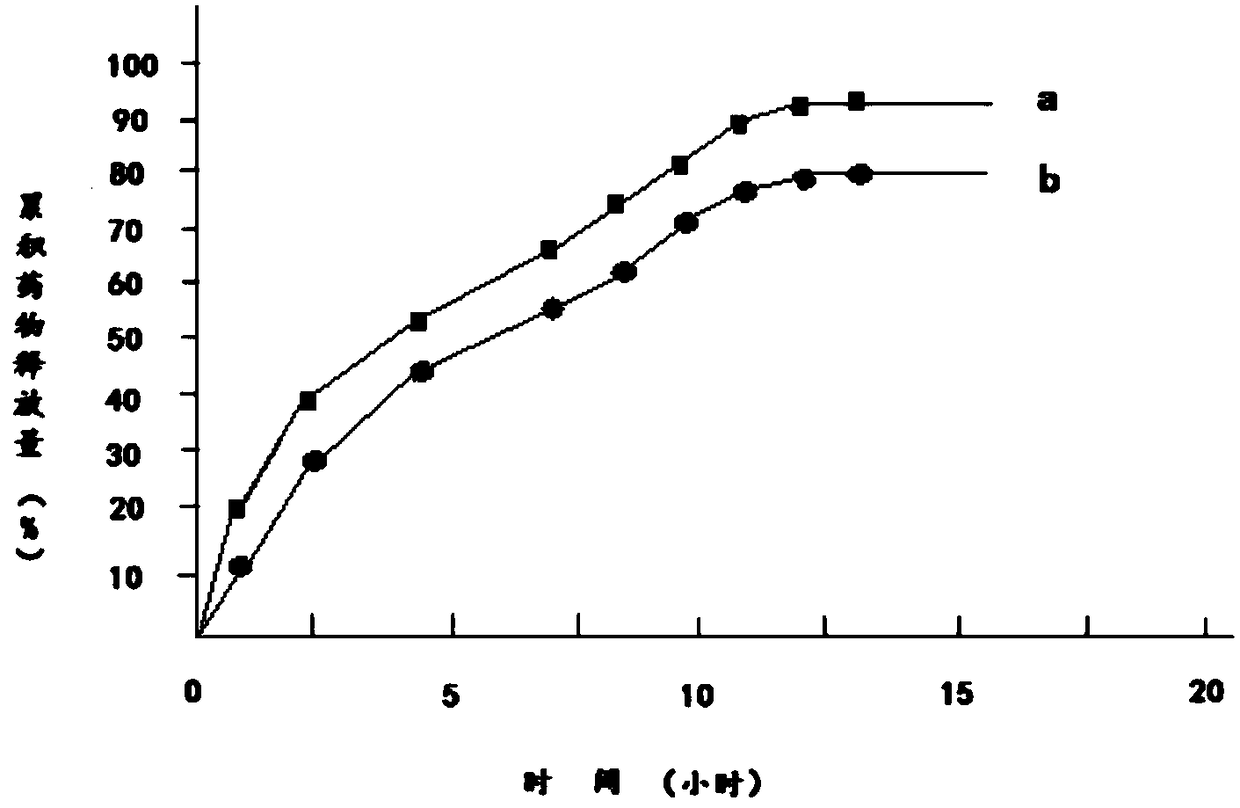
Embodiment 2
[0032] 1) Dissolve 100g of diclofenac sodium in 130g of 95% ethanol to obtain diclofenac sodium ethanol solution, which is referred to as solution A;
[0033] 2) Add 180g of hydroxypropyl β-cyclodextrin and 25g of mannitol to solution A to obtain solution B;
[0034] 3) 110g of polylactic acid and 25g of polyethylene glycol 200 were dissolved in 190g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which was designated as solution C;
[0035] 4) Mix solution B and solution C to obtain solution D;
[0036] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15°C to 18°C, continuously stir solution D for 12 hours, then reduce the temperature of solution D to 0°C to 1°C within 2 hours and let it stand for 12 hours. Hours, keep the temperature of solution D at 0°C to 1°C during the standing period;
[0037] 6) Heat the solution D obtained in step 5, and stir continuously for 12 hours when the temperature of the so...
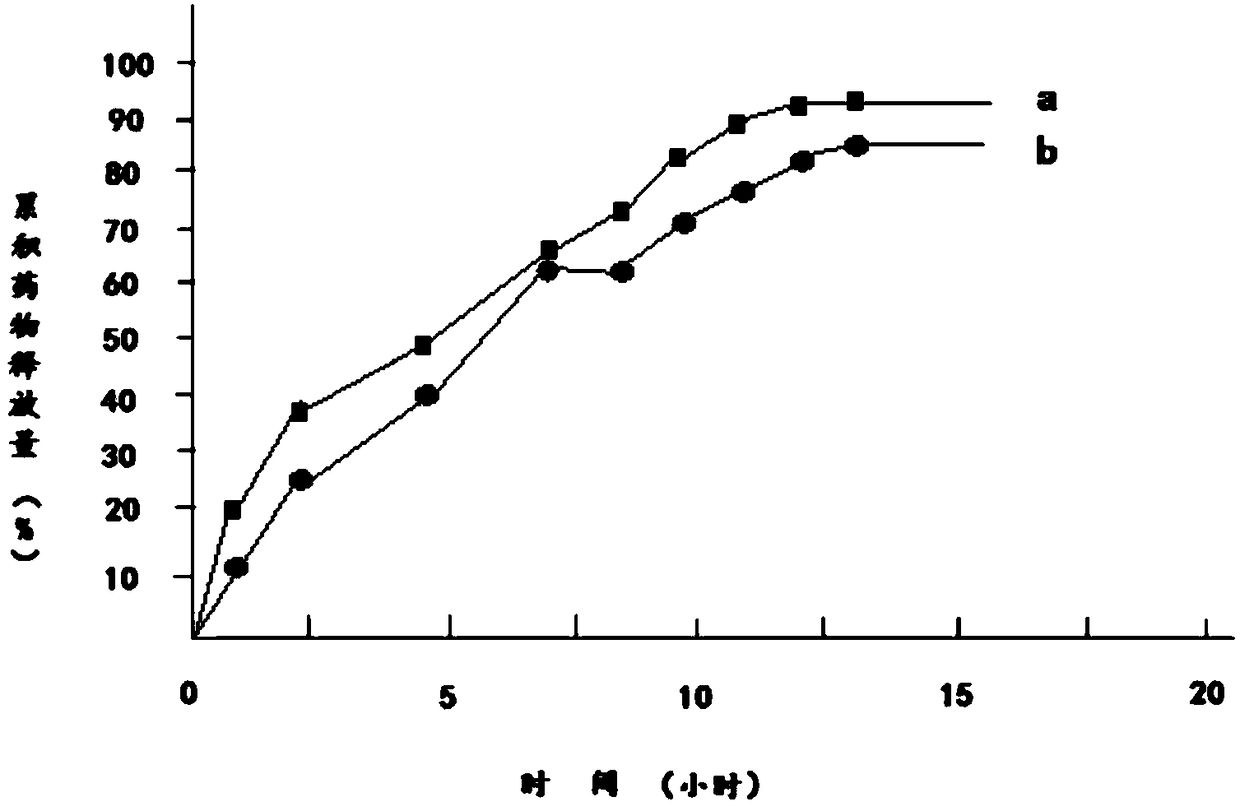
Embodiment 3
[0041] 1) Dissolve 100g of diclofenac sodium in 150g of 95% ethanol to obtain diclofenac sodium ethanol solution, which is referred to as solution A;
[0042] 2) Add 200g of hydroxypropyl β-cyclodextrin and 30g of mannitol to solution A to obtain solution B;
[0043] 3) Dissolve 120g of polylactic acid and 30g of polyethylene glycol 200 in 200g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is referred to as solution C;
[0044] 4) Mix solution B and solution C to obtain solution D;
[0045] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15°C to 18°C, continuously stir solution D for 12 hours, then reduce the temperature of solution D to 0°C to 1°C within 2 hours and let it stand for 12 hours. Hours, keep the temperature of solution D at 0°C to 1°C during the standing period;
[0046] 6) Heat the solution D obtained in step 5, and stir continuously for 12 hours when the temperature of the solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com