Preparation method of metformin hydrochloride sustained-release preparation
A technology of metformin hydrochloride and sustained-release preparations, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, and medical preparations containing active ingredients, etc., which can solve problems such as unfavorable oral administration, changes in drug release, and large amount of excipients. Good slow-release function, moderate dosage and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
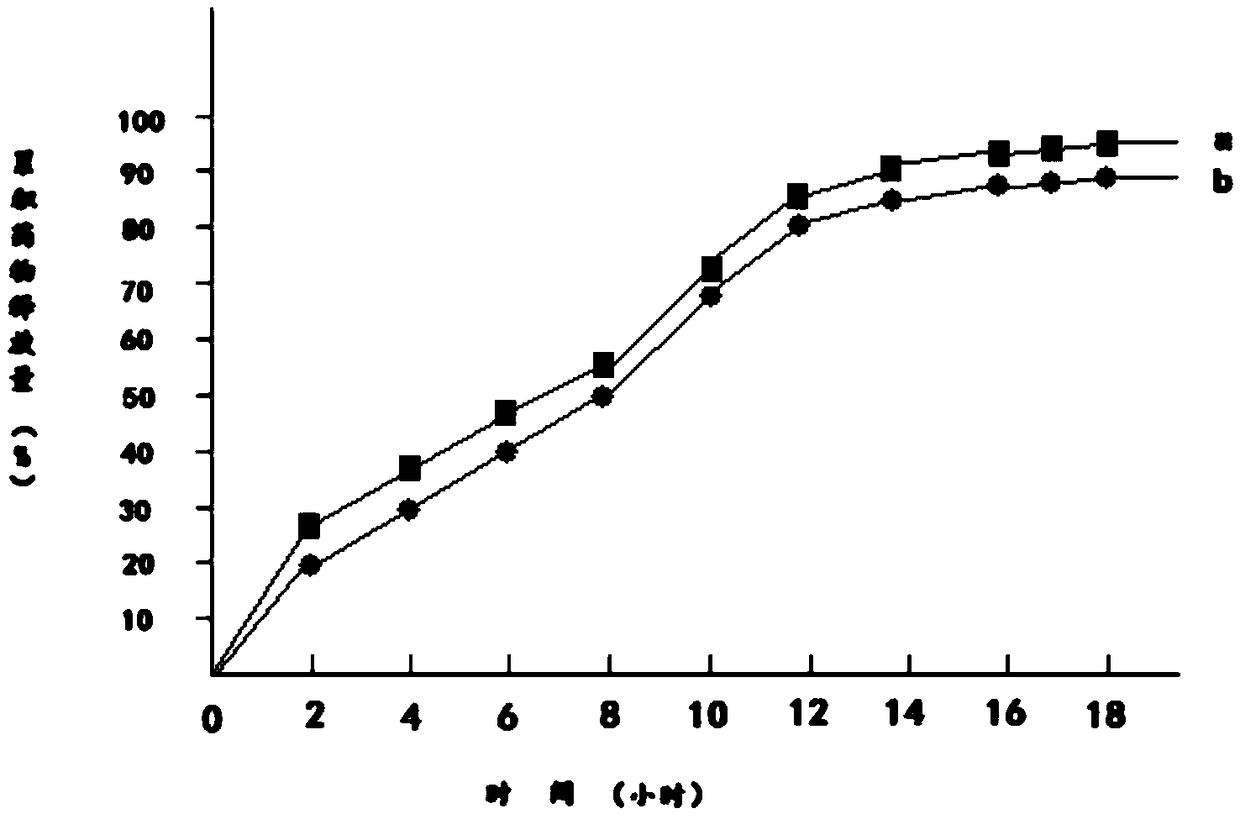
Embodiment 1
[0023] 1) 100g metformin hydrochloride is dissolved in 120g95% ethanol to obtain metformin hydrochloride ethanol solution, which is denoted as solution A;
[0024] 2) Add 100g of hydroxypropyl β-cyclodextrin and 20g of mannitol to solution A to obtain solution B;
[0025] 3) Dissolve 100g of polylactic acid and 20g of polyethylene glycol 200 in 100g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is referred to as solution C;
[0026] 4) Mix solution B and solution C to obtain solution D;
[0027] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15°C to 18°C, continuously stir solution D for 12 hours, then reduce the temperature of solution D to 0°C to 1°C within 2 hours and let it stand for 12 hours. Hours, keep the temperature of solution D at 0°C to 1°C during the standing period;
[0028] 6) Heat the solution D obtained in step 5, and stir continuously for 12 hours when the temperature of the sol...
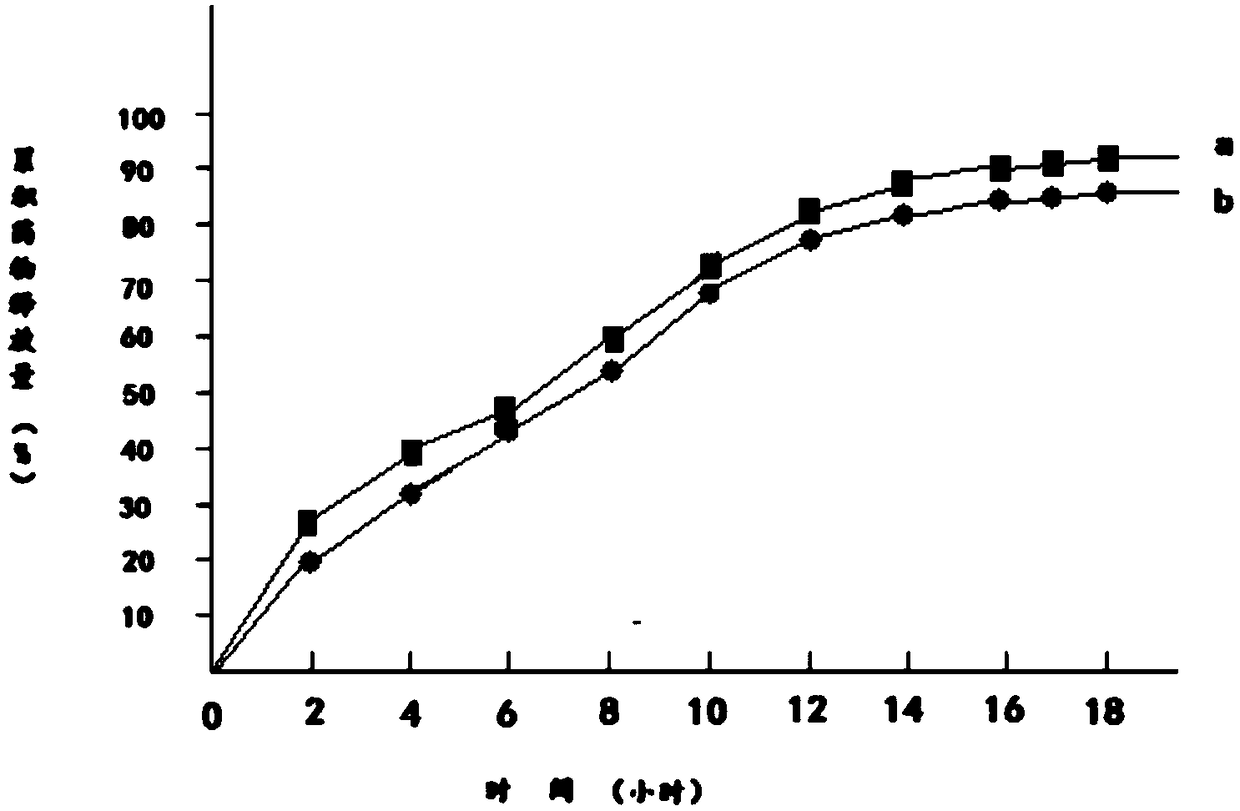
Embodiment 2
[0032] 1) 100g metformin hydrochloride is dissolved in 130g95% ethanol to obtain metformin hydrochloride ethanol solution, which is designated as solution A;
[0033] 2) Add 120g of hydroxypropyl β-cyclodextrin and 25g of mannitol to solution A to obtain solution B;
[0034] 3) Dissolve 130g of polylactic acid and 30g of polyethylene glycol 200 in 130g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is referred to as solution C;
[0035] 4) Mix solution B and solution C to obtain solution D;
[0036] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15°C to 18°C, continuously stir solution D for 12 hours, then reduce the temperature of solution D to 0°C to 1°C within 2 hours and let it stand for 12 hours. Hours, keep the temperature of solution D at 0°C to 1°C during the standing period;
[0037] 6) Heat the solution D obtained in step 5, and stir continuously for 12 hours when the temperature of the ...
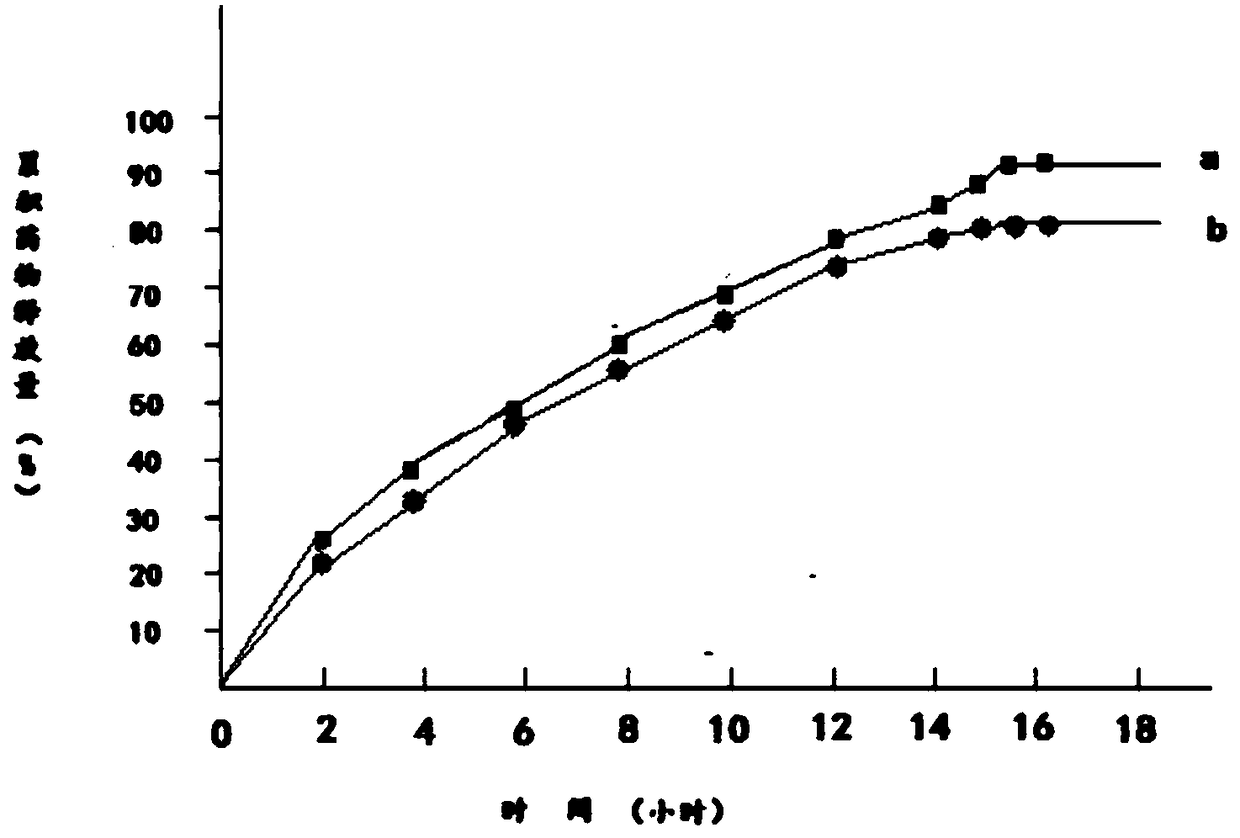
Embodiment 3
[0041] 1) 100g metformin hydrochloride is dissolved in 150g95% ethanol to obtain metformin hydrochloride ethanol solution, which is designated as solution A;
[0042] 2) Add 130g of hydroxypropyl β-cyclodextrin and 30g of mannitol to solution A to obtain solution B;
[0043] 3) Dissolve 140g of polylactic acid and 40g of polyethylene glycol 200 in 140g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is referred to as solution C;
[0044] 4) Mix solution B and solution C to obtain solution D;
[0045] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15°C to 18°C, continuously stir solution D for 12 hours, then reduce the temperature of solution D to 0°C to 1°C within 2 hours and let it stand for 12 hours. Hours, keep the temperature of solution D at 0°C to 1°C during the standing period;
[0046] 6) Heat the solution D obtained in step 5, and stir continuously for 12 hours when the temperature of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com