Preparation method of vitaminB6 sustained release preparation
A technology of sustained-release preparations and vitamins, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. Good release function, high production efficiency, and moderate dosage of capsule material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
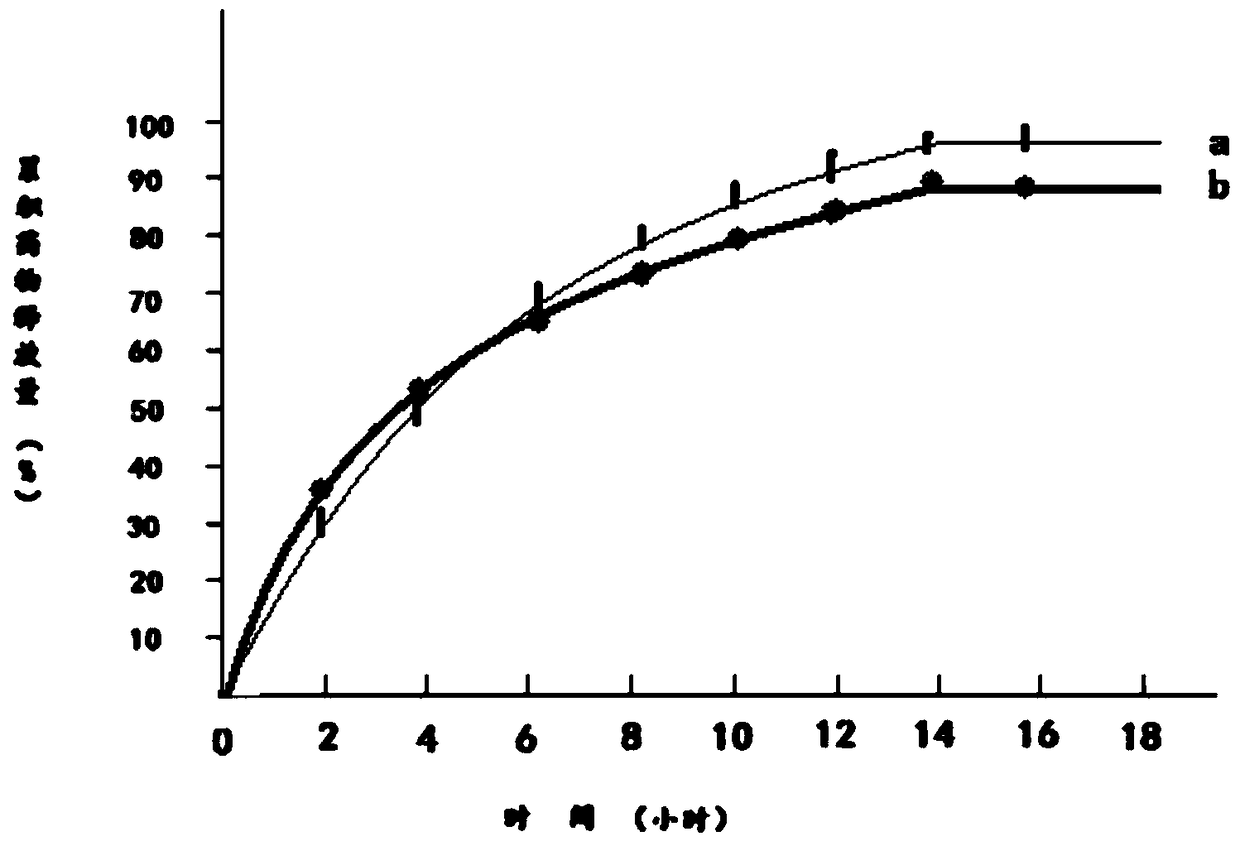
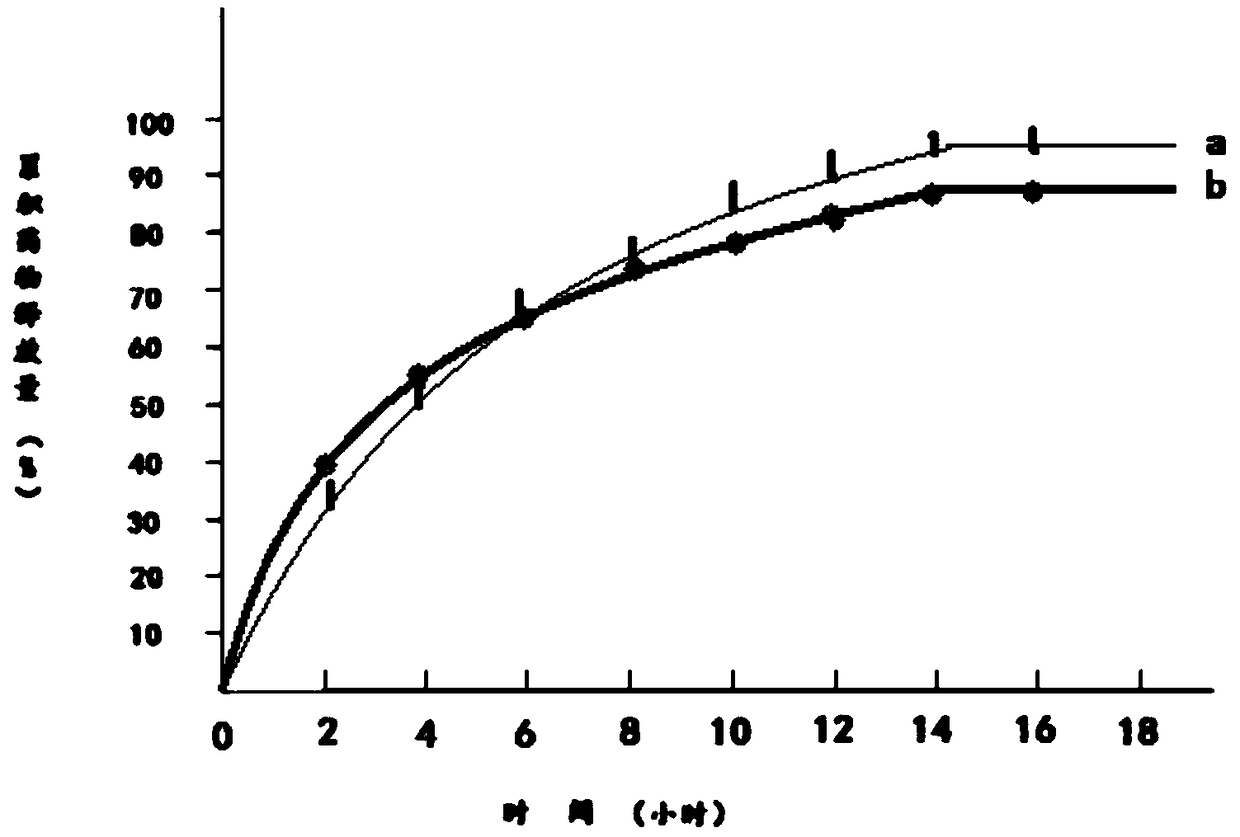
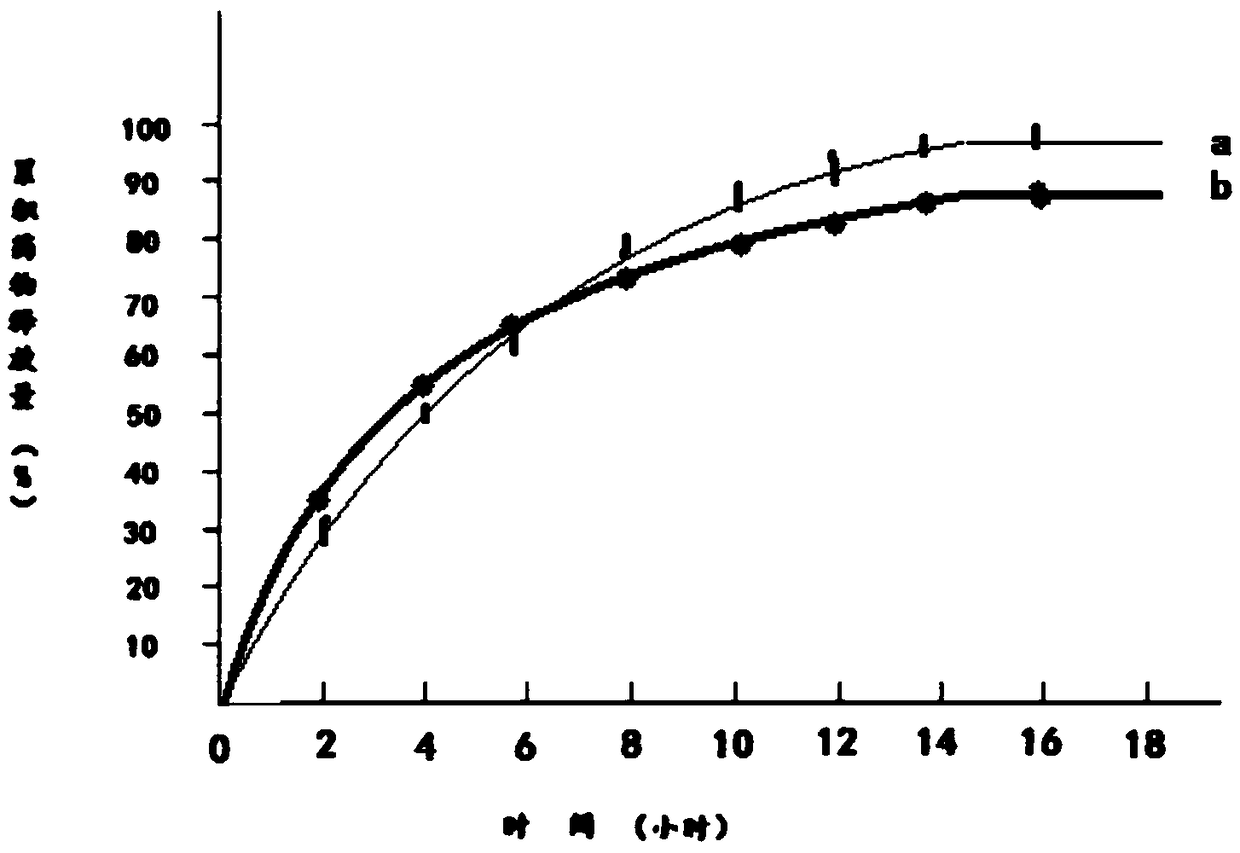
Image
Examples
Embodiment 1
[0023] 1) Dissolve 100g of vitamin B6 in 110g of 95% ethanol to obtain a vitamin B6 ethanol solution, which is denoted as solution A;
[0024] 2) Add 110g of hydroxypropyl β-cyclodextrin and 20g of mannitol to solution A to obtain solution B;
[0025] 3) Dissolve 130g of polylactic acid and 20g of polyethylene glycol 200 in 130g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is referred to as solution C;
[0026] 4) Mix solution B and solution C to obtain solution D;
[0027] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15°C to 18°C, continuously stir solution D for 12 hours, then reduce the temperature of solution D to 0°C to 1°C within 2 hours and let it stand for 12 hours. Hours, keep the temperature of solution D at 0°C to 1°C during the standing period;
[0028] 6) Heat the solution D obtained in step 5, and stir continuously for 12 hours when the temperature of the solution D rises to 15°C...
Embodiment 2
[0032] 1) Dissolve 100g of vitamin B6 in 120g of 95% ethanol to obtain a vitamin B6 ethanol solution, which is denoted as solution A;
[0033] 2) Add 120g of hydroxypropyl β-cyclodextrin and 25g of mannitol to solution A to obtain solution B;
[0034] 3) Dissolve 140g of polylactic acid and 30g of polyethylene glycol 200 in 140g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is referred to as solution C;
[0035] 4) Mix solution B and solution C to obtain solution D;
[0036] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15°C to 18°C, continuously stir solution D for 12 hours, then reduce the temperature of solution D to 0°C to 1°C within 2 hours and let it stand for 12 hours. Hours, keep the temperature of solution D at 0°C to 1°C during the standing period;
[0037] 6) Heat the solution D obtained in step 5, and stir continuously for 12 hours when the temperature of the solution D rises to 15°C...
Embodiment 3
[0041] 1) Dissolve 100g of vitamin B6 in 130g of 95% ethanol to obtain a vitamin B6 ethanol solution, which is denoted as solution A;
[0042] 2) Add 130g of hydroxypropyl β-cyclodextrin and 30g of mannitol to solution A to obtain solution B;
[0043] 3) Dissolve 150g of polylactic acid and 40g of polyethylene glycol 200 in 150g of acetone to obtain a solution of polylactic acid and polyethylene glycol 200, which is referred to as solution C;
[0044] 4) Mix solution B and solution C to obtain solution D;
[0045] 5) Transfer solution D to a magnetic stirrer, control the temperature of solution D at 15°C to 18°C, continuously stir solution D for 12 hours, then reduce the temperature of solution D to 0°C to 1°C within 2 hours and let it stand for 12 hours. Hours, keep the temperature of solution D at 0°C to 1°C during the standing period;
[0046]6) Heat the solution D obtained in step 5, and stir continuously for 12 hours when the temperature of the solution D rises to 15°C-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com