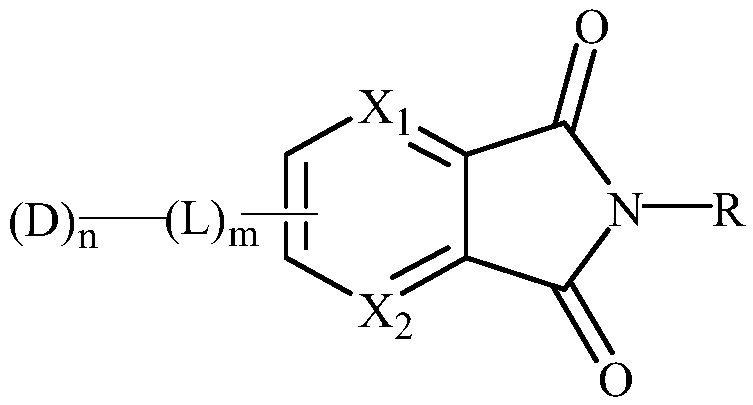
Nitrogen-doped diimide compound, and organic light-emitting display device
A technology of azadiimide and heterodiimide, which is applied in the field of organic electroluminescent materials, and can solve problems such as the difficulty in developing heavy metal doped materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091]
[0092] In a 250ml round bottom flask, 9,9-dimethylacridine borate (0.01mol), 1-bromo-3-chlorobenzene (0.012mol) and tetrakis(triphenylphosphine)palladium (0.0006mol) Add 15ml tetrahydrofuran THF, add 10ml 2M K 2 CO 3 solution, stirred at a certain speed, and the resulting mixed solution reactant was heated to reflux at a reaction temperature of 80°C for 18 hours; after the reaction was completed, it was cooled to room temperature and 100ml of water was added, and the resulting mixture was filtered and washed three times in 25ml of dichloroethane , and finally dried over anhydrous magnesium sulfate. The resulting residue was further separated and purified through a silica gel column to obtain an intermediate product M1.
[0093] In a 250ml round bottom flask, add intermediate product M1 (0.01mol), intermediate reactant A1 (0.015mol), K 2 CO 3 (0.076mol) and dimethyl sulfoxide (20ml), stirred at a certain speed, fed with nitrogen, and heated to reflux at 150°C fo...
Embodiment 2
[0096]
[0097] In a 250 ml round bottom flask, phenoxazine borate (0.01 mol), 1-bromo-3-chlorobenzene (0.012 mol) and tetrakis(triphenylphosphine)palladium (0.0006 mol) were added to 15 ml of tetrahydrofuran THF, Add 10ml of 2M K 2 CO 3 solution, stirred at a certain speed, and the resulting mixed solution reactant was heated to reflux at a reaction temperature of 80°C for 18 hours; after the reaction was completed, it was cooled to room temperature and 100ml of water was added, and the resulting mixture was filtered and washed three times in 25ml of dichloroethane , and finally dried over anhydrous magnesium sulfate. The resulting residue was further separated and purified through a silica gel column to obtain an intermediate product M5.
[0098] In a 250ml round bottom flask, add intermediate product M5 (0.01mol), intermediate reactant A5 (0.012mol), K 2 CO 3 (0.076mol) and dimethyl sulfoxide (20ml), stirred at a certain speed, fed with nitrogen, and heated to reflux...
Embodiment 3
[0101]
[0102] In a 250ml round bottom flask, carbazole borate (0.02mol), 2-chloro-4,6-dibromobenzene (0.012mol) and tetrakis(triphenylphosphine) palladium (0.0006mol) were added to 15ml tetrahydrofuran THF , add 10ml 2M K 2 CO 3 solution, stirred at a certain speed, and the resulting mixed solution reactant was heated to reflux at a reaction temperature of 80°C for 18 hours; after the reaction was completed, it was cooled to room temperature and 100ml of water was added, and the resulting mixture was filtered and washed three times in 25ml of dichloroethane , and finally dried over anhydrous magnesium sulfate. The resulting residue was further separated and purified through a silica gel column to obtain an intermediate product M14.
[0103] In a 250ml round bottom flask, add intermediate product M14 (0.02mol), intermediate reactant A14 (0.025mol), K 2 CO 3 (0.076mol) and dimethyl sulfoxide (20ml), stirred at a certain speed, fed with nitrogen, and heated to reflux at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com