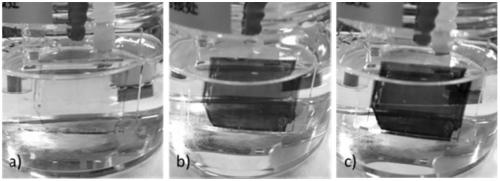
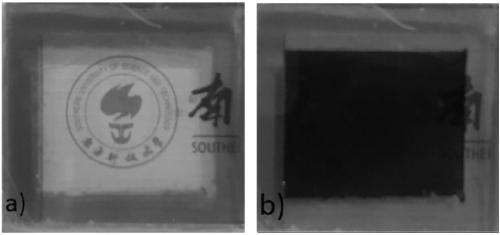
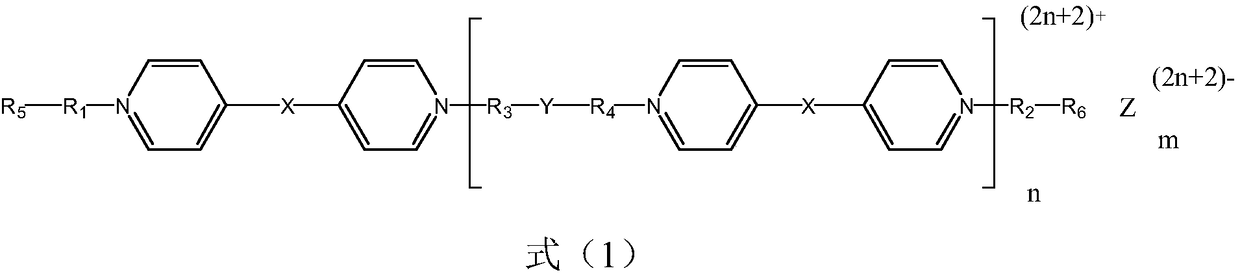
Electrochromic compound and application thereof, electrochromic device manufactured by using electrochromic compound and application of electrochromic device
An electrochromic device and electrochromic technology are applied in the field of electrochromic devices and electrochromic compounds, which can solve the problems of short color changing response time and low preparation cost, and achieve wide color changing range and high light transmittance difference. , Improve the effect of radiation resistance and temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Compound I was prepared by the following steps:
[0056] Step (1), 4-pyridineboronic acid pinacol ester (2.01g, 9.8mmol), tetrakis (triphenylphosphine) palladium (407mg, 0.352mmol), potassium phosphate (5.95g, 28mmol) was added in the 250ml flask, Add 70mL of 1,4-dioxane solution containing 2,5-dibromothiophene (394μL, ρ=2.15mg / mL, 847mg, 3.61mmol) in a nitrogen atmosphere, heat to 90°C, react for 72h, and the reaction is complete After the reaction solution was cooled to room temperature, the filter residue was washed with chloroform, the filtrate was spin-dried in a rotary evaporator, and finally purified by silica gel column chromatography (the eluent was a mixed solution with a mass ratio of chloroform:methanol=200:1) to obtain a yellow solid Namely compound 1 (575mg), yield 69%;
[0057] Step (2), the compound 1 (476mg, 2mmol) obtained in step (1) and diethyl bromoethylphosphate (490mg, 2mmol) were dissolved in 10mL DMF, heated to 90°C, after 24h of reaction, the ...
Embodiment 2
[0066] Compound II was prepared by the following steps:
[0067] Step (1), 3,4-ethylenedioxythiophene (1.42g, 10mmol) was dissolved in 30mL DMF, and cooled to 0°C with an ice bath, N-bromosuccinimide (3.78g, 21mmol) was dissolved In 30mL of DMF, slowly drop into the above solution with a constant pressure dropping funnel. After the dropwise addition, the reaction solution was stirred at room temperature for 2h. After the reaction, 100mL of ice water was slowly added to the reaction solution to produce a large amount of light yellow solid , the product was filtered under reduced pressure, and the filter residue was washed three times with 15 mL of deionized water to obtain 2.85 g of the product, namely compound 4, with a yield of 95%;
[0068] Step (2), 4-pyridineboronic acid pinacol ester (2.01g, 9.8mmol), tetrakis (triphenylphosphine) palladium (407mg, 0.352mmol), potassium phosphate (5.95g, 28mmol) was added in a 250mL flask, Add 70mL of 1,4-dioxane solution containing comp...
Embodiment 3
[0078] The only difference from Example 1 is that compound 8 is used instead of 2,5-dibromothiophene in step (1), and the molar amount of compound 8 added is the same as that of 2,5-dibromothiophene.
[0079] Compound 8 has the following structure:
[0080]
[0081] Embodiment 3 obtains compound III, and compound III has the following structure:
[0082]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com