Composite, preparation method and application thereof in lithium battery electrolyte
A compound and electrolyte technology, applied in the application, preparation and compound field of lithium battery electrolyte, can solve the problems of poor temperature tolerance, poor voltage resistance, flammability and explosion, and achieve high flash point, Good voltage resistance and high safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
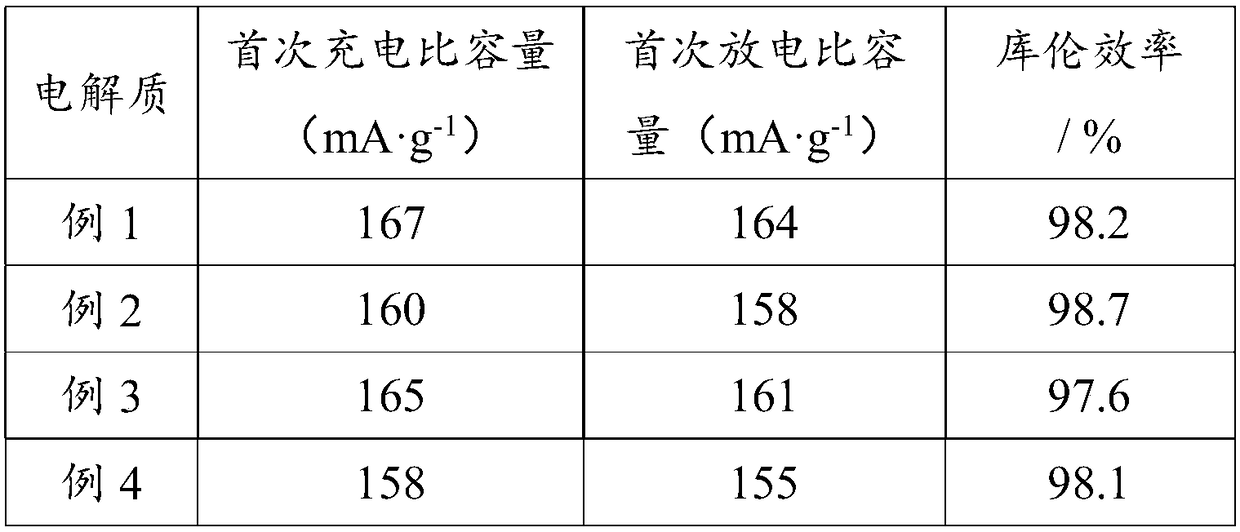
Embodiment 1
[0025] This example is a specific example of preparing the electrolyte product 1.
[0026] In this example, the ether-functionalized pyrrolidine ionic liquid 1 is 1-methyl-1-ethyl ether pyrrolidine bis(trifluoromethylsulfonyl)imide salt, and the anion 1 is bis(trifluoromethyl Sulfonyl) imide anion, cation 1 is 1-methyl-1-ethyl ether pyrrolidine cation. Among them, the preparation method of ether-functionalized pyrrolidine ionic liquid 1 is as follows: 4 g of 1-methylpyrrolidine and 7.5 g of bromoethyl ethyl ether are weighed, dissolved in acetonitrile, heated in an oil bath, and the reaction does not exceed 24 hours. After the reaction, the acetonitrile was spun off to obtain 1-methyl-1-ethyl ether pyrrolidine bromide. Then 1-methyl-1-ethyl ether pyrrolidinium bromide and LiTFSI were dissolved in 40mL ultrapure water at a molar ratio of 1:1.1, stirred at room temperature for 4h, and the ionic liquid phase and the aqueous phase were separated, and the ionic liquid phase was se...
Embodiment 2
[0029] This example is a specific example of preparing the electrolyte product 2.
[0030] In this example, the ether-functionalized pyrrolidine ionic liquid 2 is 1-methyl-1-ethyl ether pyrrolidine tetrafluoroborate, the anion 2 is tetrafluoroborate anion, and the cation 2 is 1-methyl- 1-Ethyl ether pyrrolidine cation. Among them, the preparation method of the ether-functionalized pyrrolidine ionic liquid 2 is as follows: 3 g of 1-methylpyrrolidine and 5.5 g of bromoethyl ethyl ether are weighed, dissolved in acetonitrile, heated in an oil bath, and the reaction does not exceed 24 hours. After the reaction, the acetonitrile was spun off to obtain 1-methyl-1-ethyl ether pyrrolidine bromide. Then 1-methyl-1-ethyl ether pyrrolidine bromide and LiBF 4 Dissolve in 40mL ultrapure water at a molar ratio of 1:1.1, stir at room temperature for 4h, separate the ionic liquid phase and the water phase, wash the ionic liquid phase with ultrapure water five times, add ethyl acetate, then ...
Embodiment 3
[0033] This example is a specific example of preparing the electrolyte product 3 .
[0034] In this embodiment, the ether-functionalized pyrrolidine ionic liquid 3 is 1-methyl-1-ethyl ether pyrrolidine bis(fluorosulfonimide) salt, and the anion 3 is bis(fluorosulfonimide) ) anion, and cation 3 is 1-methyl-1-ethyl ether pyrrolidine cation. Among them, the preparation method of ether-functionalized pyrrolidine ionic liquid 3 is as follows: weigh 6 g of 1-methylpyrrolidine and 11 g of bromoethyl ethyl ether, dissolve in acetonitrile, heat in an oil bath, and react for no more than 24 hours. After the reaction, the acetonitrile was spun off to obtain 1-methyl-1-ethyl ether pyrrolidine bromide. Then 1-methyl-1-ethyl ether pyrrolidinium bromide and LiFSI were dissolved in 40mL ultrapure water at a molar ratio of 1:1.1, stirred at room temperature for 4h, and the ionic liquid phase and the aqueous phase were separated, and the ionic liquid phase was separated into Wash five times w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com