Diquinoxaline phenazine derivative and synthesis method and application thereof
A kind of bisquinoxaline phenazine and quinoxaline phenazine technology, applied in the field of bisquinoxaline phenazine derivatives and synthesis thereof, can solve problems such as low intrinsic conductivity, achieve low cost, good reproducibility, The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
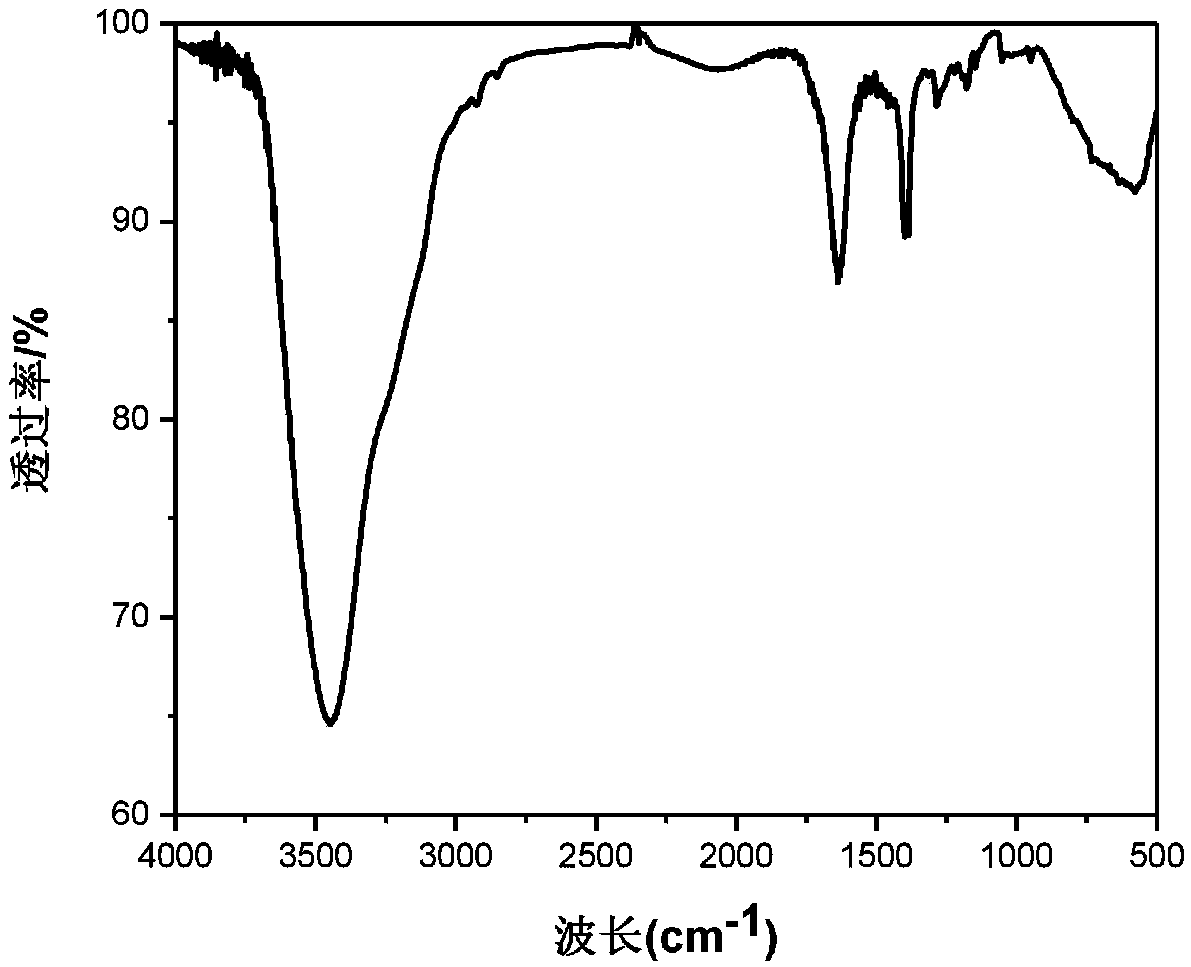
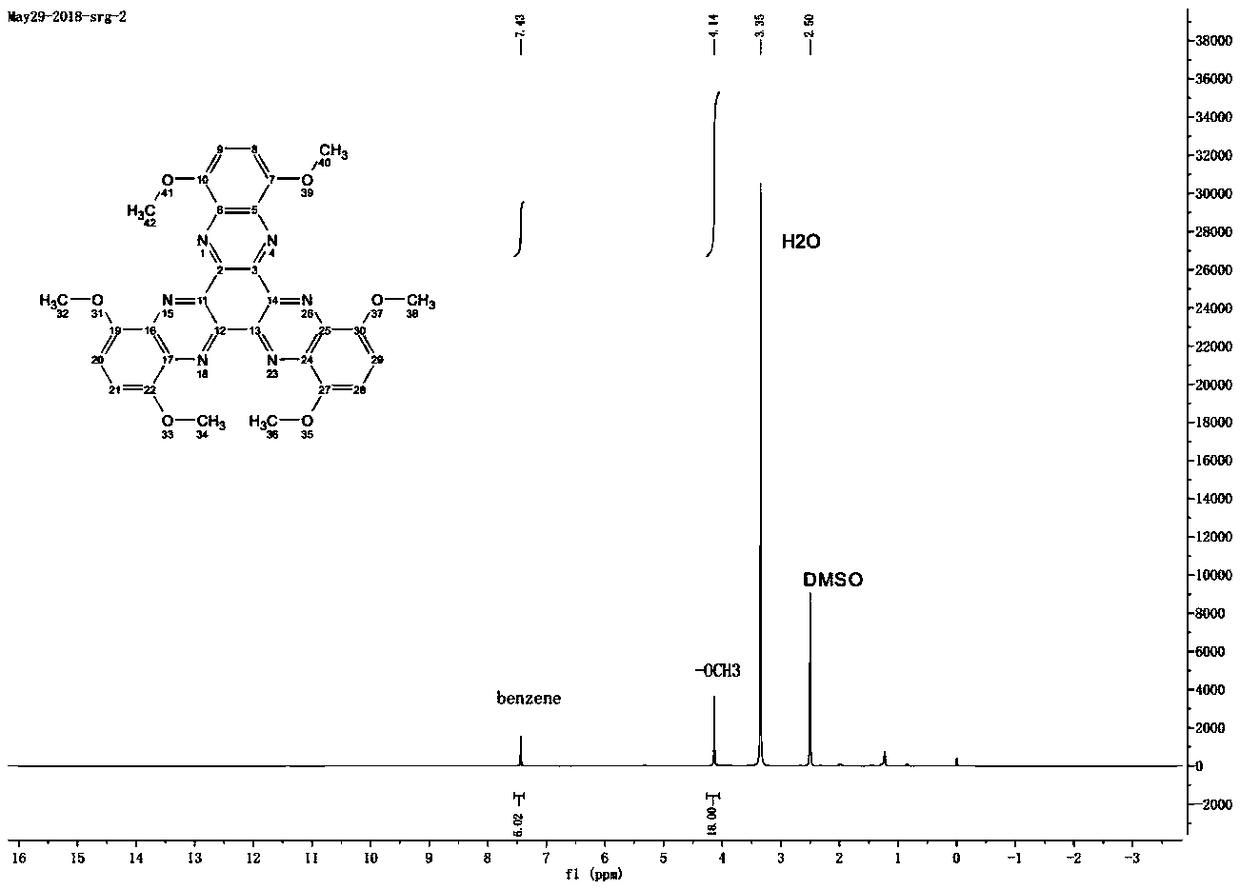
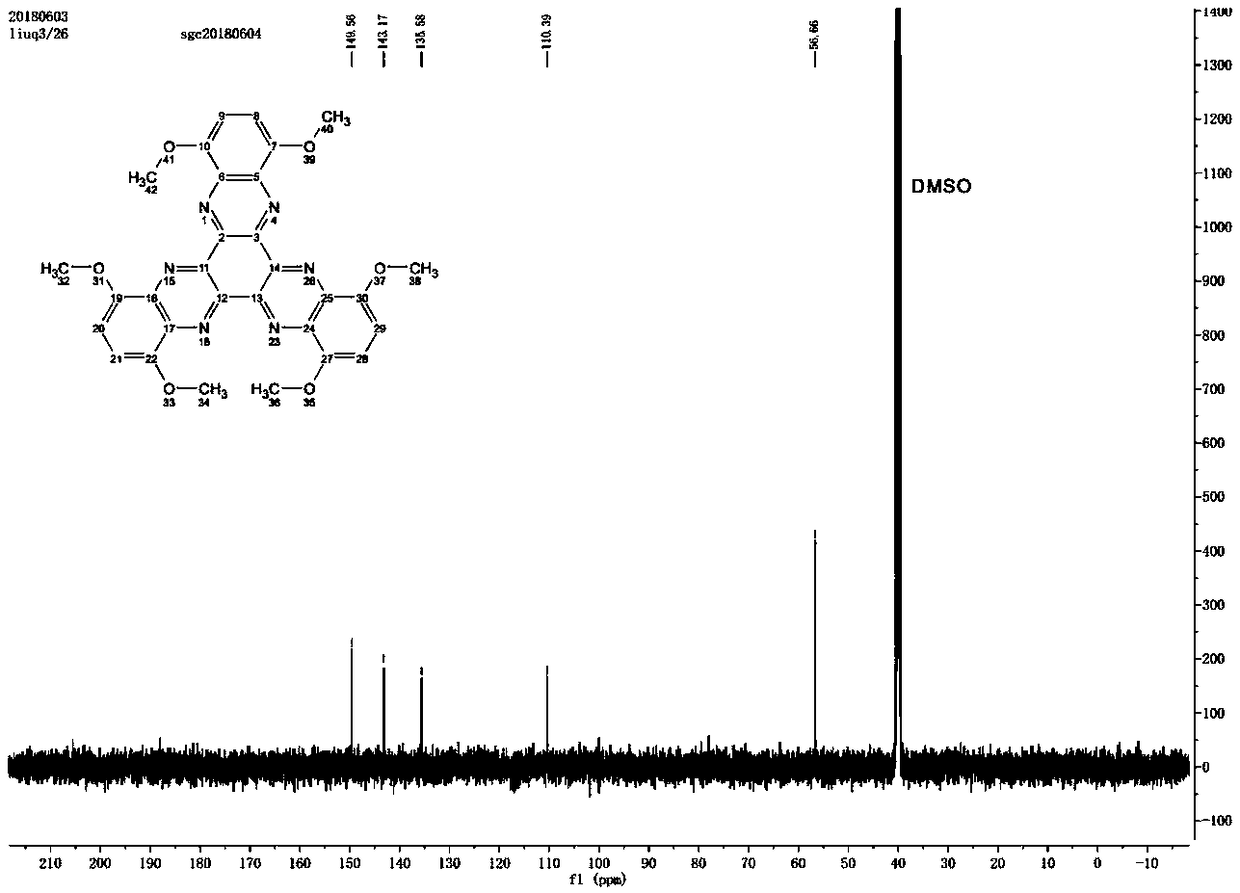
[0029] In a 100mL three-necked flask, 60mL of acetic acid was added, while 2,3-diamino-1,4-dimethoxybenzene (0.756g, 4.5mmol), cyclohexanone decahydrate (0.522g, 1.5mmol) were added , under the protection of nitrogen, the reaction mixture was continuously stirred in the reflux state. The reaction time was 48 hours. After the reaction, stop heating, add water to precipitate the brown solid product, collect the solid by suction filtration, wash with water and ethanol for several times, and vacuum at 80 ° C. After drying for 6h, Compound A: 1,4,7,10,13,16-hexamethoxydiquinoxaline phenazine was obtained. figure 1 is the infrared spectrum, figure 2 is the H NMR spectrum, image 3 is the carbon NMR spectrum, Figure 4 for the mass spectrogram.
[0030] 1 H NMR(DMSO,500MHz),δ:7.43(s,6H),4.14(s,18H)
[0031] 13 C NMR (DMSO, 500MHz), δ: 149.56, 143.17, 135.58, 110.39, 56.66
[0032] ESI-MS m / z: C 30 h 24 N 6 o 6 Calculated value: 565.56[M+H] + :565.25
[0033] FT-IR (KBr,...
Embodiment 2
[0035] The experimental method is the same as in Example 1, except that 2,3-diamino-1,4,-dimethoxybenzene is changed to 2,3-diamino-1,4,-diethoxybenzene (0.892g, 4.5 mmol), to obtain compound B: 1,4,7,10,13,16-hexaethoxydiquinoxaline phenazine.
Embodiment 3
[0037] The experimental method is the same as in Example 1, except that 2,3-diamino-1,4,-dimethoxybenzene is changed to 2,3-diamino-p-phenol (0.64g, 4.5mmol) to obtain compound C: 1 ,4,7,10,13,16-Hexahydroxydiquinoxaline phenazine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com