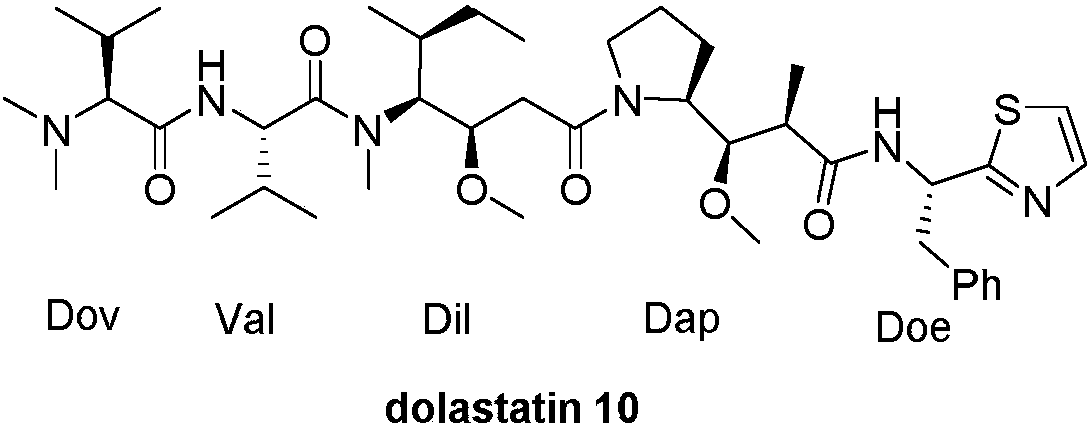
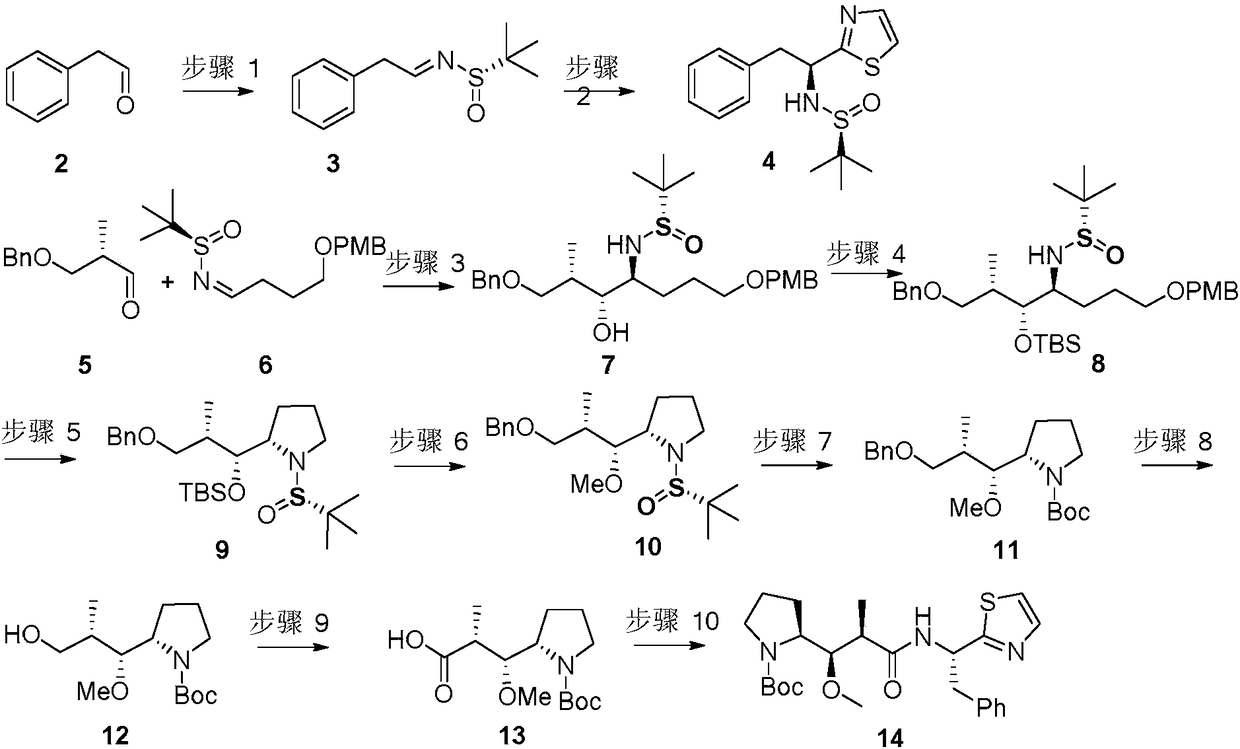
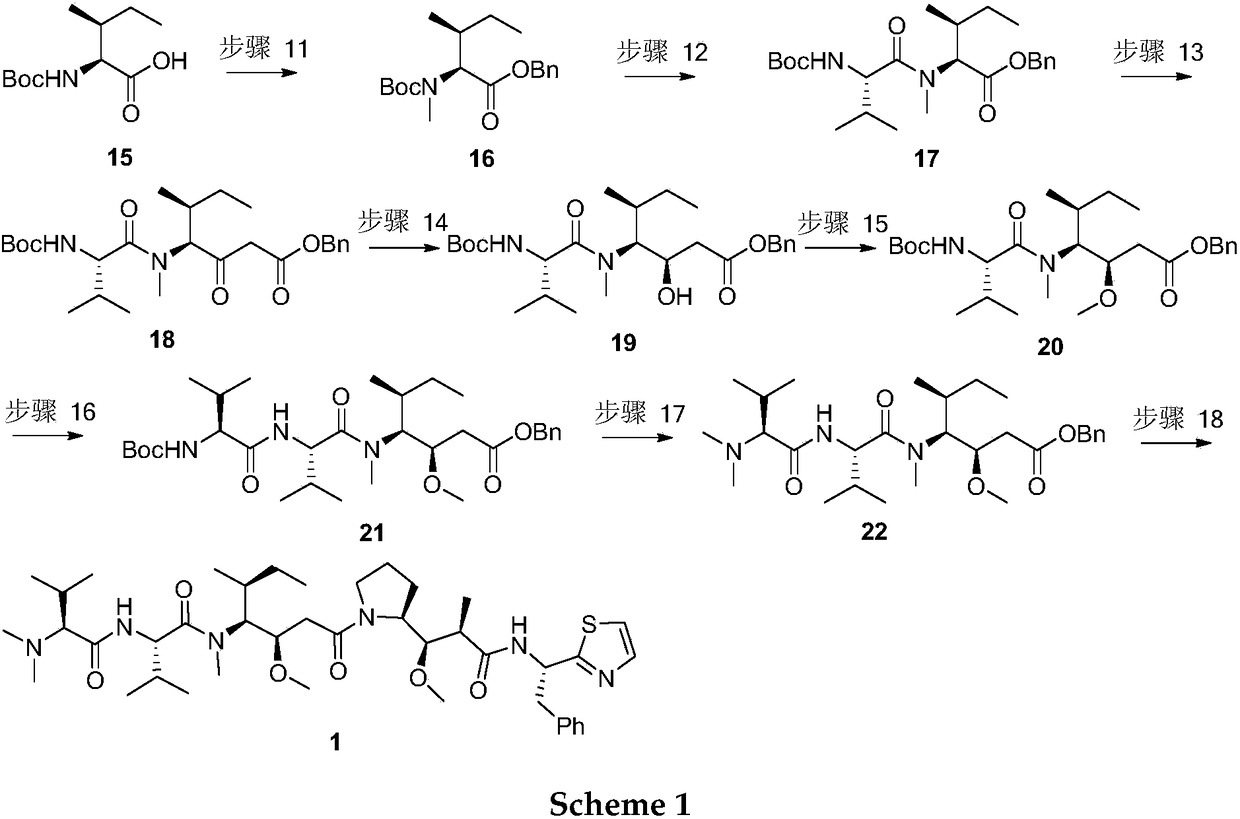
Method for preparing Dolastatin 10 having high anticancer activity
A technology of dolastatin and anti-cancer activity, applied in the direction of peptides, etc., can solve the problems that hinder the research on the structure of dolastatin 10, the synthesis is difficult, and there are not many routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of compound 3: Dissolve compound 2 (5.00g, 41.61mmol) in 150mL of dichloromethane, add (S)-tert-butylsulfinamide (5.55g, 45.77mmol), anhydrous copper sulfate (13.28g, 83.22mmol) and pyridine p-toluenesulfonate (1.05g, 4.16mmol), stirred at room temperature for 36 hours, concentrated by filtration, and purified by silica gel column to obtain light yellow liquid 3 (7.43g, 80%);
[0035] Synthesis of compound 4: Dissolve 2-bromothiazole (2.42mL, 26.87mmol) in 90mL of toluene, lower to -78 degrees Celsius, slowly add n-butyllithium in hexane (1.6M in hexane, 22.39mmol), stir for 30 Minutes later, compound 3 was dissolved in 10 mL of toluene and added slowly, and reacted at -78 degrees Celsius for three hours, quenched by saturated ammonium chloride solution, extracted with ethyl acetate, dried, concentrated, and purified on a silica gel column to obtain a colorless liquid 4 (3.66g, 53%), 1 H NMR (CDCl 3 ,400MHz):δ7.76(d,J=3.2Hz,1H),7.28-7.19(m,5H),7.13-7.10(m,2H...
Embodiment 2
[0053] Synthesis of compound 18: Dissolve compound 17 (8.00g, 18.41mmol) and 10% palladium carbon (800mg) in 500mL methanol, stir for 5 hours under the action of hydrogen, filter, concentrate and dissolve in 70mL tetrahydrofuran, add chloroformic acid Ethyl ester (2.00g, 18.41mmol), stirred at room temperature for 2 hours and then set aside; Benzyl acetate (8.43mL, 58.91mmol) was dissolved in 70mL tetrahydrofuran, cooled to -78 degrees Celsius, slowly added lithium diisopropylamide ( 27.62mL, 55.23mmol), after stirring for 30 minutes, add the activated ester prepared above, stir at -78 degrees Celsius for 3 hours, add saturated ammonium chloride solution to quench, extract with ethyl acetate, dry, filter, concentrate, and Silica gel column purification gave colorless liquid 18 (1.76 g, 20%).
Embodiment 3
[0055] Synthesis of compound 18: Dissolve compound 17 (8.00g, 18.41mmol) and 10% palladium carbon (800mg) in 500mL of methanol, stir for 5 hours under the action of hydrogen, filter, concentrate and dissolve in 70mL of tetrahydrofuran, add N, N'-carbonyldiimidazole (2.99g, 18.41mmol), stirred at room temperature for 2 hours and then set aside; Benzyl acetate (8.43mL, 58.91mmol) was dissolved in 70mL of tetrahydrofuran, cooled to -78 degrees Celsius, slowly added diisopropyl Lithium amide (27.62mL, 55.23mmol), after stirring for 30 minutes, the activated ester prepared above was added, stirred at -78°C for 3 hours, then quenched by adding saturated ammonium chloride solution, extracted with ethyl acetate, dried, filtered, Concentrate and purify by silica gel column to give colorless liquid 18 (7.02 g, 80%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com