Conditioned cell immortalization lentiviral vector, construction method and application thereof in establishment of porcine granulosa cell line
A technology of a lentiviral vector and a construction method, which is applied to the lentiviral vector and its construction method and the application field in the porcine ovary granulosa cell line, can solve the problems of difference, lack of granulosa cell line, inability to produce inhibin and in vitro differentiation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A method for constructing a conditional cell immortalized lentiviral vector, comprising the steps of:
[0036] 1) According to the molecular cloning technique, the Tet-on-3G lentiviral vector (Addgene, 50661) was double digested with Nhe1 and Bamh1, and the 7595bp vector fragment was recovered;
[0037] 2) Using primer 1 (sequence shown in SEQ ID NO1) and primer 2 (sequence shown in SEQ ID NO2), using pLVX-Large T-EF1-EGFP as a template to amplify the Large T-T2A fragment with a length of 2155bp ; Using primer 3 (sequence shown in SEQ ID NO3) and primer 4 (sequence shown in SEQ ID NO4), using pLVX-sgRNA-mCherry as a template to amplify the T2A-mCherry fragment, the length is 774bp;
[0038] 3) Using the Large T-T2A fragment and the T2A-mCherry fragment obtained in step 2) as templates, adding primer 1 and primer 4, performing overlapping PCR amplification, and obtaining a DNA fragment with a length of 2875 bp of the LargeT-T2A-mCHRRY fragment;
[0039] 4) Recover throu...
Embodiment 2
[0042] A method for constructing a reversible and inducible porcine ovary granulosa cell line, comprising the steps of:
[0043] 1) Isolation and cultivation of granulosa cells:
[0044] Pig ovary samples were obtained from a commercial slaughterhouse, brought back to the laboratory in warm sterile saline (38°C) within 2 h, and then transferred to the laboratory. Use a 10mL syringe to absorb the oocytes and ovarian granulosa cells in the follicular fluid, then remove the oocytes and the complex of oocytes and granulosa cells with a mouth pipette, collect the porcine ovarian granulosa cells, and centrifuge at 600g for 5 minutes to collect the cells and wash 3 times, grow to 10cm 2 In a petri dish, the culture system uses DMEM (Gibco, 11960044)) + 10% fetal bovine serum (Gibco, 10099-141) + 1% GlutaMAX TM (Gibco, 35050061)+20ng / mL epidermal growth factor (Peprotech, AF-100-15) to obtain primary porcine ovarian granulosa cells;
[0045] 2) Lentivirus packaging and cell infecti...
Embodiment 3
[0050] Example 3: GCs isolation and infection of Large T gene
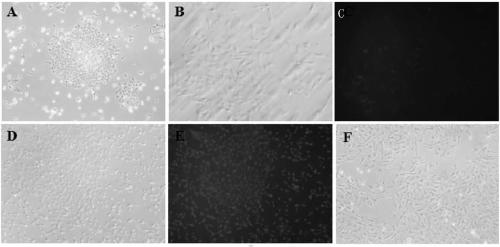
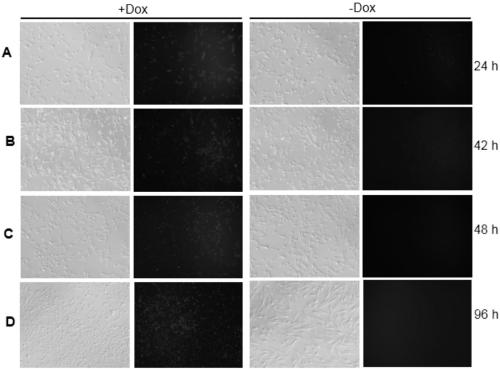
[0051] The morphology of GCs isolated and cultured in the present invention is short and fibrous (such as figure 2 Shown in A), by pLVX-Tet3G-Large T-T2A-mCherry-Puro r Lentiviral transfection, while adding Dox induction, showed that GCs cell transgenes (such as figure 2 Shown in B and 2C); by adding puromycin for selection, the Large T gene was successfully transfected, and the positive rate of the cells reached 100% to express mCherry, while maintaining the morphology and rapid proliferation of fibroblasts, continuously cultured in vitro for more than 6 months (such as figure 2 Shown in D and 2E), while the non-transgenic primary cells, cultured to the 5th day, the morphology began to change obviously, and the refraction and state became worse (such as figure 2 F shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com