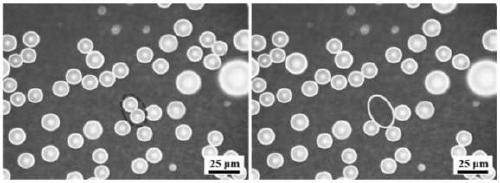
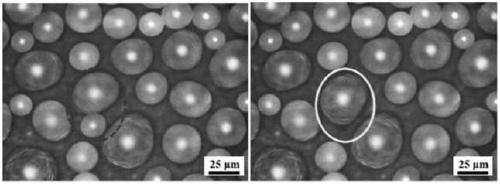
Preparation method for copper based super hydrophobic surface with condensation liquid droplet self-bounce characteristic
A super-hydrophobic surface, condensate technology, applied to special surfaces, devices for coating liquids on surfaces, coatings, etc., to achieve the effect of simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Wash the surface of the copper substrate with ethanol and deionized water in sequence, and dry it with nitrogen gas for later use.
[0022] (2) Apply a constant DC current to the above-mentioned cleaned copper substrate in the electrolyte (wherein, the cleaned copper substrate is used as the cathode, and the platinum sheet is used as the anode), at 0.01A / cm 2 Under the current density, the electrodeposition treatment is performed for 300 seconds; wherein, the electrolyte is 1 mol of nickel chloride and 0.5 mol of boric acid added to each liter of aqueous solution containing ammonium chloride additive. The ammonium chloride additive concentration in the aqueous solution of the above-mentioned ammonium chloride additive is 1.5mol / L.
[0023] (3) The electrodeposited copper substrate was placed in a closed container containing 15 microliters of 1H,1H,2H,2H-perfluorodecyltriethoxysilane and heated for modification. The heating temperature was set to 120°C, and the heati...
Embodiment 2
[0026] (1) Wash the surface of the copper substrate with ethanol and deionized water in sequence, and dry it with nitrogen gas for later use.
[0027] (2) Apply a constant DC current to the above-mentioned cleaned copper substrate in the electrolyte (wherein, the cleaned copper substrate is used as the cathode, and the platinum sheet is used as the anode), at 0.01A / cm 2 Under the current density, the electrodeposition process lasts for 900 seconds; wherein, the electrolytic solution is 1 mol of nickel chloride and 0.5 mol of boric acid added to each liter of aqueous solution containing ammonium chloride additive. The ammonium chloride additive concentration in the aqueous solution of the above-mentioned ammonium chloride additive is 1.5mol / L.
[0028] (3) The electrodeposited copper substrate was placed in a closed container containing 15 microliters of 1H,1H,2H,2H-perfluorodecyltriethoxysilane and heated for modification. The heating temperature was set to 120°C, and the heat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com