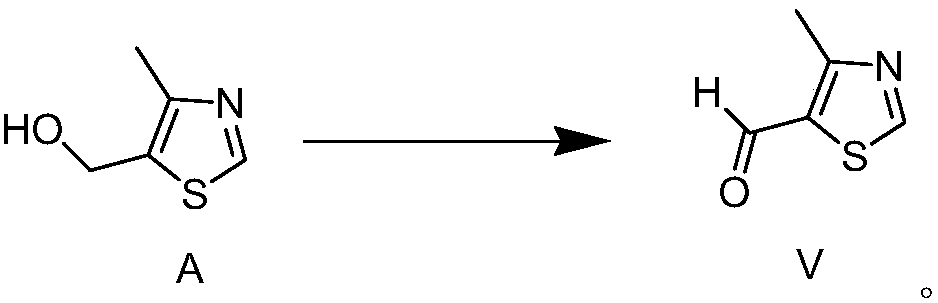
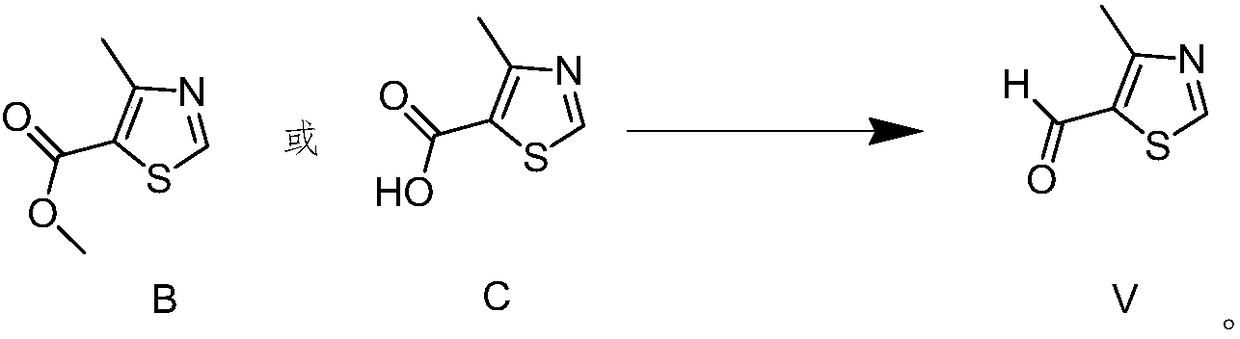
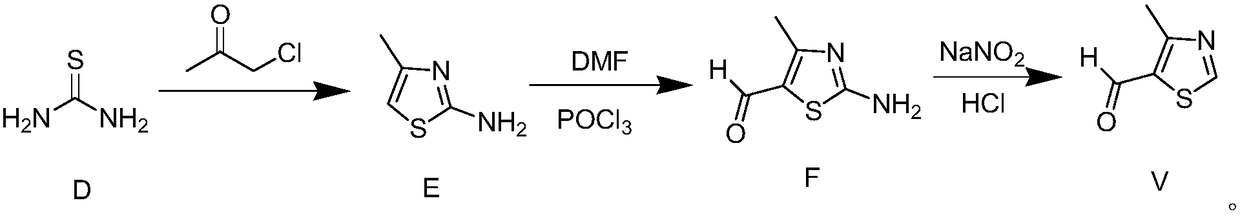
Novel preparation method for 4-methylthiazole-5-carboxaldehyde
A technology of methyl thiazole and formyl thiazole, applied in the direction of organic chemistry, etc., can solve the problems of difficult treatment, large amount of waste water, waste salt, less application, etc., and achieve the effects of improving yield, mild conditions, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of 2-aminomethyl-4-methylthiazole (G)
[0042]In the 200L enamel reaction kettle, add 18kg 2-amino-4-methylthiazole, add 80kg process water, 9.0kg caustic soda, turn on stirring cooling, turn on interlayer freezing and cooling; keep the inner temperature at about -5 ℃, start to drip 23kg Dimethyl sulfate, control the dripping speed, keep the internal temperature at about 0 °C, and finish dripping within 2 hours. After dripping, the reaction was incubated for 3 hours. The temperature was raised to about 25-30°C, and the reaction was maintained for 1 hour. Pump 25kg of ethyl acetate into the kettle to extract for the first time. After standing for 0.5 hours, the layers were separated, and the lower aqueous phase was taken for a second time with 15 kg of dichloromethane. The organic phases were combined and washed once with 10 kg of process water. After standing for 0.5 hours, the layers were separated, and the aqueous phase was separated. The organic phas...
Embodiment 2
[0048] This example is the same as Example 1, except that: in the preparation of 4-methyl-5-thiazole carboxaldehyde (V), a 10% Pd / C catalyst with a moisture content of 5% is used, and the yield is 51%.
Embodiment 3
[0050] This example is the same as Example 1, except that: in the preparation of 4-methyl-5-thiazole carboxaldehyde (V), a 10% Pd / C catalyst with a moisture content of 3% is used, and the yield is 27%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com