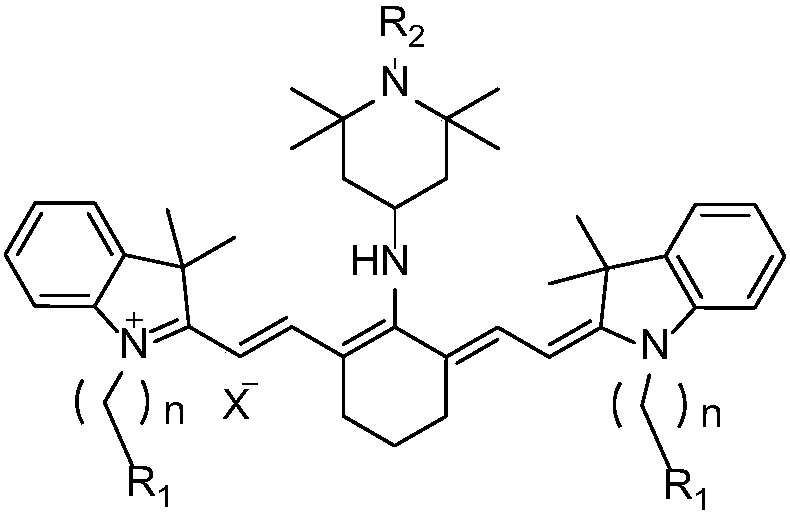
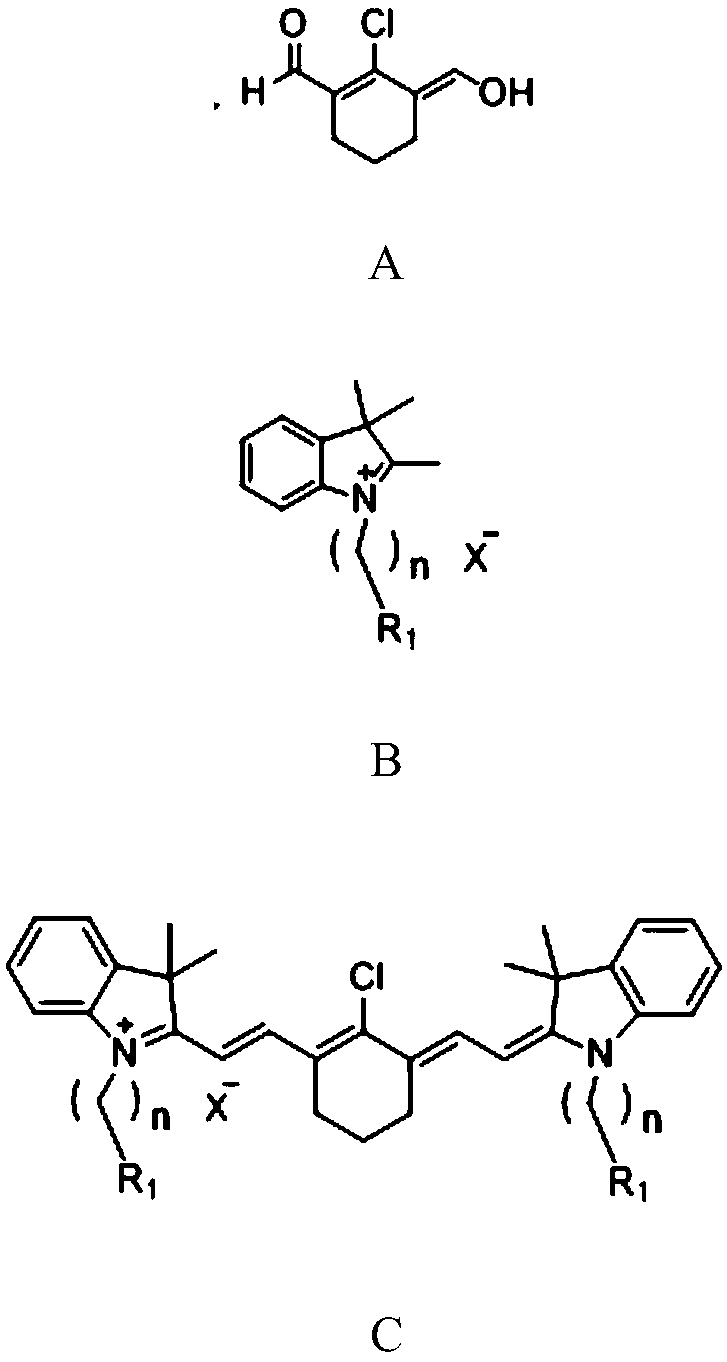
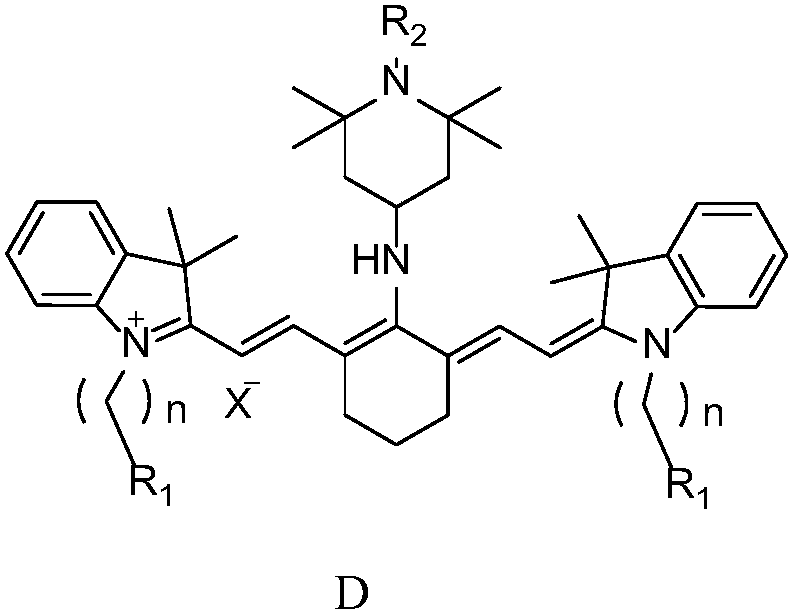
Preparation method of near-infrared cyanine dye
A cyanine dye and near-infrared technology, which is applied in the field of preparation of near-infrared cyanine dyes, can solve the problems of reduced performance, limited practical application, poor photostability, etc., and achieves the effect of improving photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of near-infrared cyanine dyes with good photostability, R 1 for hydrogen, R 2 is hydrogen, X - For iodide ion, n=1.
[0033] Synthesis of Compound A:
[0034]
[0035] Add N,N-dimethylformamide (14.62g, 200mmol) into a 250mL two-neck round-bottomed flask, cool in an ice-salt bath until the solution temperature is below 5°C, and add phosphorus oxychloride (15.33g, 100 mmol) was slowly added dropwise into a round bottom flask, and the temperature of the solution was controlled to maintain at 0-5°C. After stirring for 10 minutes, add cyclohexanone (9.81g, 100mmol) dropwise into the round bottom flask, control the temperature of the solution at 0-5°C, remove the ice bath and then stir for 10 minutes, then raise the temperature to 70°C for 2 hours . After the reaction solution was cooled to room temperature, it was slowly poured into 500 mL of ice-water mixture, and a yellow solid was precipitated, which was allowed to stand overnight. Suction filtration...
Embodiment 2
[0047] Preparation of near-infrared cyanine dyes with good photostability, R 1 for hydrogen, R 2 is methyl, X - For iodide ion, n=1.
[0048] Synthesis of Compound A:
[0049]
[0050] Add N,N-dimethylformamide (54.82g, 750mmol) into a 250mL two-necked round-bottomed flask, cool in an ice-salt bath until the solution temperature is below 5°C, and add phosphorus oxychloride (23.00g, 150 mmol) was slowly added dropwise into a round bottom flask, and the temperature of the solution was controlled to maintain at 0-5°C. After stirring for 15 minutes, add cyclohexanone (4.91g, 50mmol) dropwise into the round bottom flask, control the temperature of the solution at 0-5°C, remove the ice bath and stir for 15 minutes, then raise the temperature to 60°C for 6 hours . After the reaction solution was cooled to room temperature, it was slowly poured into 500 mL of ice-water mixture, and a yellow solid was precipitated, which was allowed to stand overnight. Suction filtration obtai...
Embodiment 3
[0062] Preparation of near-infrared cyanine dyes with good photostability, R 1 for hydrogen, R 2 is methoxy, X - For iodide ion, n=1.
[0063] Synthesis of Compound A:
[0064]
[0065] Add N,N-dimethylformamide (36.55g, 500mmol) into a 250mL two-necked round-bottomed flask, cool in an ice-salt bath until the solution temperature is below 5°C, and add phosphorus oxychloride (15.33g, 100 mmol) was slowly added dropwise into a round bottom flask, and the temperature of the solution was controlled to maintain at 0-5°C. After stirring for 20 minutes, add cyclohexanone (9.81g, 100mmol) dropwise into the round bottom flask, control the temperature of the solution at 0-5°C, remove the ice bath and stir for 30 minutes, then raise the temperature to 70°C for 8 hours . After the reaction solution was cooled to room temperature, it was slowly poured into 500 mL of ice-water mixture, and a yellow solid was precipitated, which was allowed to stand overnight. Suction filtration obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com