Method for detecting nosiheptide contents in nosiheptide fermentation broth
A technology of Nosiheptide and fermentation liquid, which is applied in the field of detection of Nosiheptide content in Nosiheptide fermentation liquid, which can solve the problems of inability to monitor the quality of the fermentation production process, inability to accurately guide production regulation, and complicated preparation of mixed reagents. To achieve the effect of convenient filtering operation, saving time and simplifying the processing steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
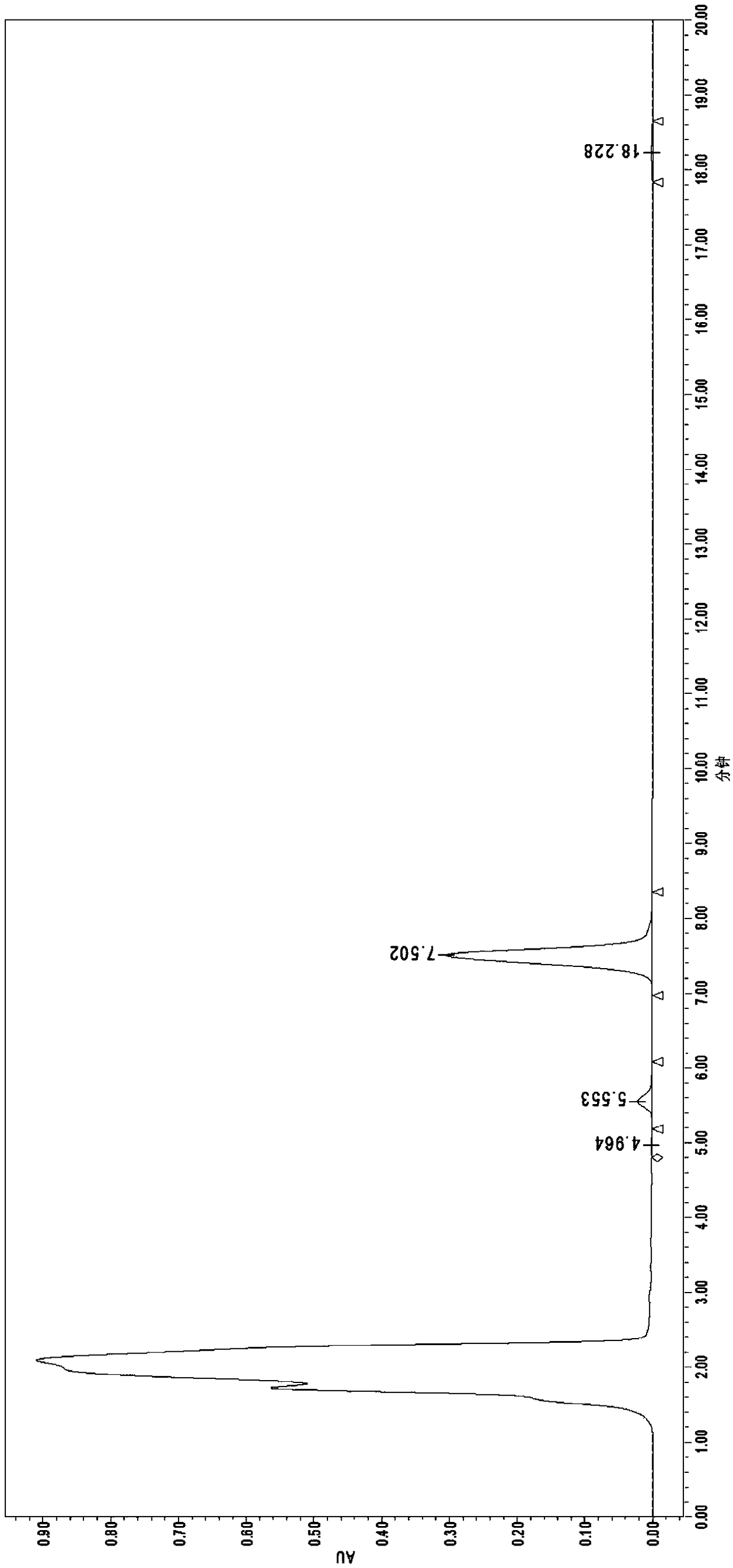
[0058] a. Preparation of Nosiheptide Reference Substance Solution: Accurately weigh 25 mg of Nosiheptide Reference Substance, dissolve it in DMF to a constant volume in a 25 mL brown volumetric flask, shake well, and use it as a stock solution. Accurately pipette 2.5mL stock solution into a 25mL brown volumetric flask, add methanol to dilute to volume, and use it as the reference solution;
[0059] b. Preparation of nosiheptide sample solution: draw 2.5mL uniform sample into a 25mL brown volumetric flask, add extraction solution (volume ratio: ethanol / water=90 / 10) to constant volume, shake well, ultrasonically extract for 25min and then shake well , let it stand for 15 minutes, take the supernatant and filter it with a 0.45 μm organic filter membrane to obtain the sample solution to be tested;
[0060] c. Sample measurement and result calculation:
[0061] Chromatographic conditions:
[0062] Liquid chromatograph: Waters e2695-2489;
[0063] Chromatographic column: C18 5.0μ...
Embodiment 2
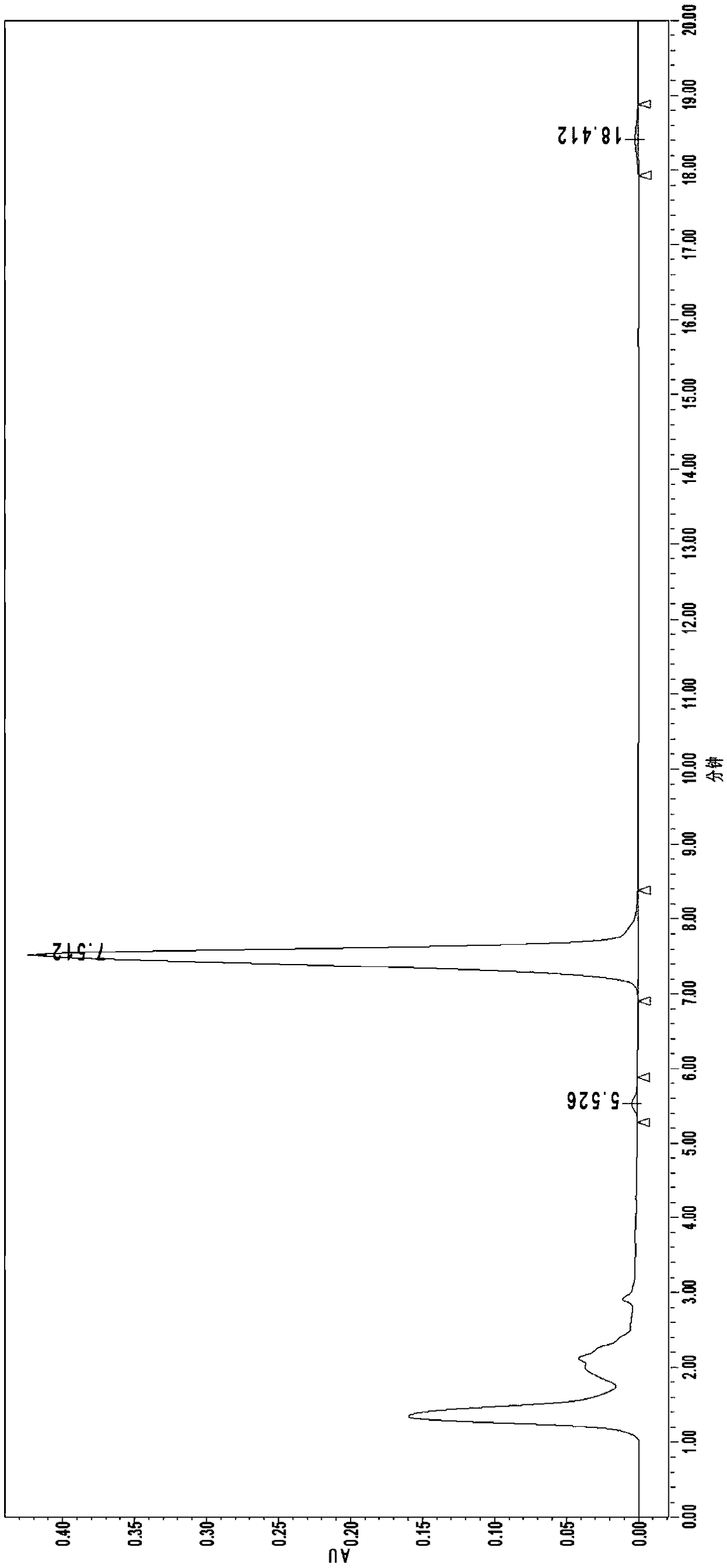
[0077] a. Preparation of Nosiheptide Reference Substance Solution: Accurately weigh 50 mg of Nosiheptide Reference Substance, dissolve it in DMF to a constant volume in a 25 mL brown volumetric flask, shake well, and use it as a stock solution. Accurately pipette 1.0mL stock solution into a 25mL brown volumetric flask, add ethanol to dilute to volume, and use it as the reference solution;
[0078] b. Preparation of nosiheptide sample solution: draw 10.0mL uniform sample into a 50mL brown volumetric flask, add extraction solution (volume ratio: ethanol / water=75 / 25) to constant volume, shake well, ultrasonically extract for 30min and then shake well , let stand for 20 minutes, take the supernatant and filter it with a 0.45 μm organic filter membrane to obtain the sample solution to be tested;
[0079] c. Sample measurement and result calculation:
[0080] Chromatographic conditions:
[0081] Liquid chromatograph: Waters 1515-2489;
[0082] Chromatographic column: C18 5.0μm 4....
Embodiment 3
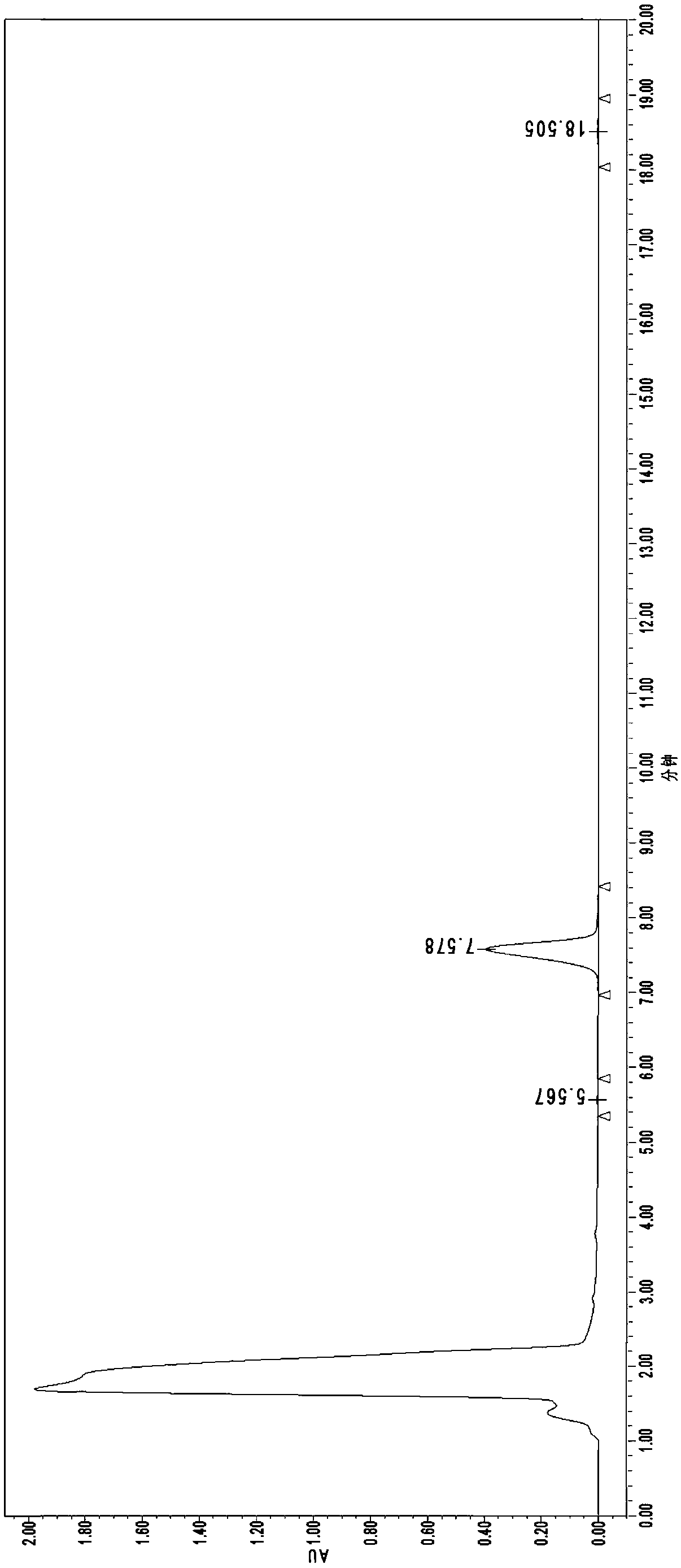
[0096] a. Preparation of Nosiheptide Reference Substance Solution: Accurately weigh 30 mg of Nosiheptide Reference Substance, dissolve it in DMF to a constant volume in a 25 mL brown volumetric flask, shake well, and use it as a stock solution. Accurately pipette 2.0mL stock solution into a 25mL brown volumetric flask, add acetonitrile to dilute to volume, and use it as the reference solution;
[0097] b. Preparation of nosiheptide sample solution: draw 2.5mL uniform sample into a 50mL brown volumetric flask, add extract solution (concentration 95% ethanol) to constant volume, shake well, shake well after ultrasonic leaching for 20min, let it stand for 10min, take The supernatant was filtered with a 0.45 μm organic filter to obtain the sample solution to be tested;
[0098] c. Sample measurement and result calculation:
[0099] Chromatographic conditions:
[0100] Liquid chromatograph: Diane U3000;
[0101] Chromatographic column: C18 5.0μm 4.6*150mm;
[0102] Column tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com