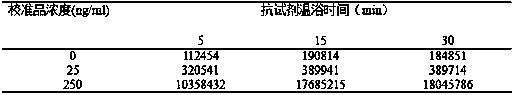Kit for detecting osteocalcin, and using method thereof
A technology of osteocalcin and kits, applied in the direction of biological testing, measuring devices, material inspection products, etc., can solve the problems of high price, unfavorable popularization, large economic burden of patients, etc., achieve good stability, improve popularization, use, and variation small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A test kit for detecting osteocalcin, characterized in that it includes a calibration product, a quality control product, a solution of a biotin-labeled osteocalcin antibody, a solution of a horseradish peroxidase-labeled osteocalcin antibody, streptavidin Avidin-labeled nano magnetic particle suspension, substrate solution and washing solution;
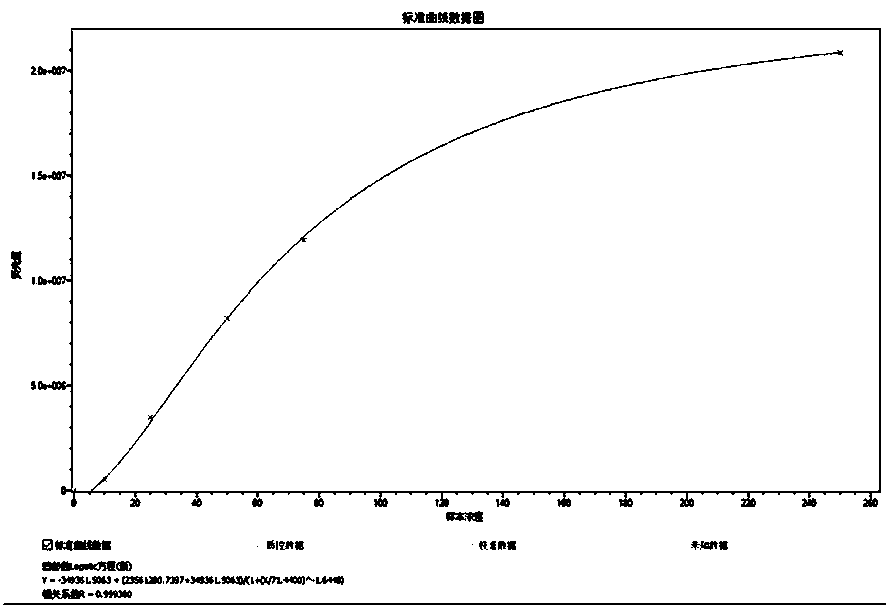
[0034] The calibrators include osteocalcin antigen solutions with concentrations of 0 ng / mL, 10 ng / mL, 25 ng / mL, 50 ng / mL, 75 ng / mL, and 250 ng / mL, and the osteocalcin antigen was purchased from sigma, the product number is O5761; the calibrator is prepared from osteocalcin antigen and 0.1M Tris-HCl buffer with a pH value of 7.2;
[0035] The quality control products include osteocalcin antigen solutions with a concentration of 25 ng / mL and 75 ng / mL, and the osteocalcin antigen is purchased from sigma, and the article number is O5761; the quality control products are composed of osteocalcin antigen and 0.1M, Tris-HCl buffer s...
Embodiment 2
[0047] Prepare different concentrations of biotin-labeled antibody and horseradish peroxidase-labeled antibody, group the biotin-labeled antibody and horseradish peroxidase-labeled antibody according to the concentration ratio, and measure the osteocalcin concentration as follows: The luminescence values at 0, 25, and 250ng / ml are shown in Table 1.
[0048] Table 1: Effect of different concentrations of anti-reagent concentration on luminescence value
[0049]
[0050] Result evaluation: When the concentrations of biotin-labeled antibody and horseradish peroxidase-labeled antibody are 1.0 μg / ml and 1.0 μg / ml respectively, the ratio of the luminescence value of the high-concentration and low-concentration calibrator is the largest, so this concentration ratio is selected as Working concentration for subsequent experiments.
[0051] According to the screened optimal concentration of anti-reagent, the effects of different sample volumes and different incubation times on the...
Embodiment 3
[0058] The method for detecting osteocalcin using the kit of the above-mentioned embodiment 1 comprises the following steps:
[0059] (1) Add 40 μL of the serum to be tested or calibrator, 60 μL of biotin-labeled osteocalcin antibody, and 50 μL of streptavidin-labeled magnetic nanoparticles to the test tube, mix well, and incubate at 37°C for 15 Minutes, the osteocalcin in the sample is captured and fixed on the surface of the nano-magnetic particles; then under the action of an external magnetic field, let it stand for 2 minutes, the magnetic particles settle down, remove the supernatant, add 500 μL of cleaning solution, remove the magnetic field, shake to make Magnetic particle resuspension; repeat this washing step 3 times to remove unbound biotin-labeled osteocalcin to obtain the first solution;
[0060] (2) Add 60 μL of horseradish peroxidase-labeled osteocalcin antibody solution to the first solution, mix well, and incubate at 37°C for 15 minutes; then, under the action ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com