Nitric oxide generating formulations and kits
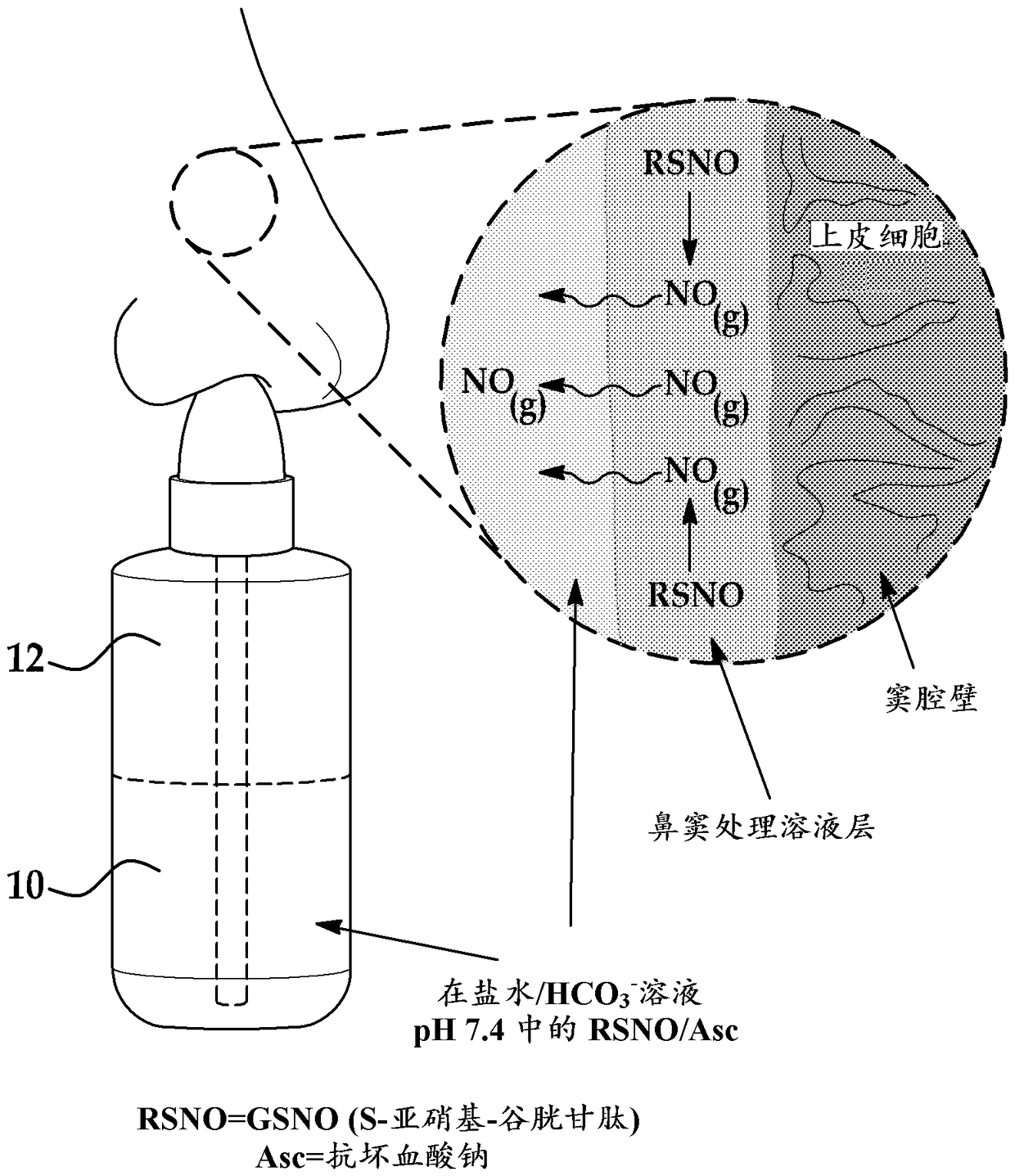
A kit and nitric oxide technology, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, catheters, etc., can solve problems such as reducing immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
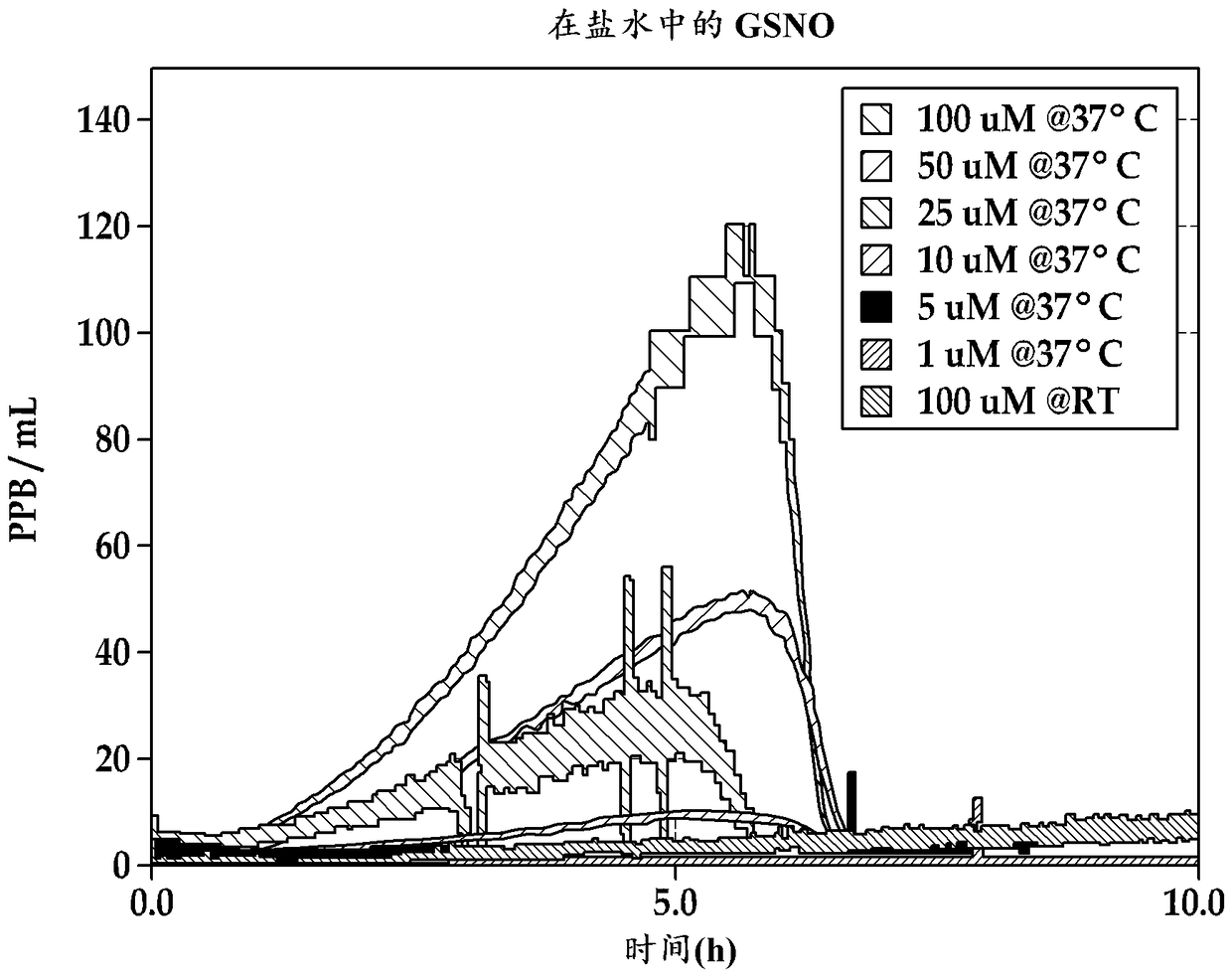
[0073] Prepare two different solutions, one with 10 µM GSNO and one with 25 µM GSNO, in phosphate-buffered saline at physiological pH 7.4. These solutions did not include the additives disclosed herein.
[0074] The NO release profiles of the two solutions were measured at 37 °C by chemiluminescence detection and the results are shown in image 3 middle. As shown, both solutions produced NO continuously for more than 7 hours, and the solution containing the higher concentration of GSNO produced higher levels of NO. It should be noted that the NO levels observed for these solutions in this example, typically in the range of 5 ppbv to 40 ppbv, were compared with the gas levels actually observed in the sinus cavity by purging the released NO into the chemiluminescent NO analyzer with The test solution, which is continuously purged with a stream of nitrogen, is significantly diluted.
[0075] In 0.16M NaCl / 0.03M NaHCO 3 Two additional solutions, one with 100 µM GSNO and one wi...
Embodiment 2
[0078] preparation
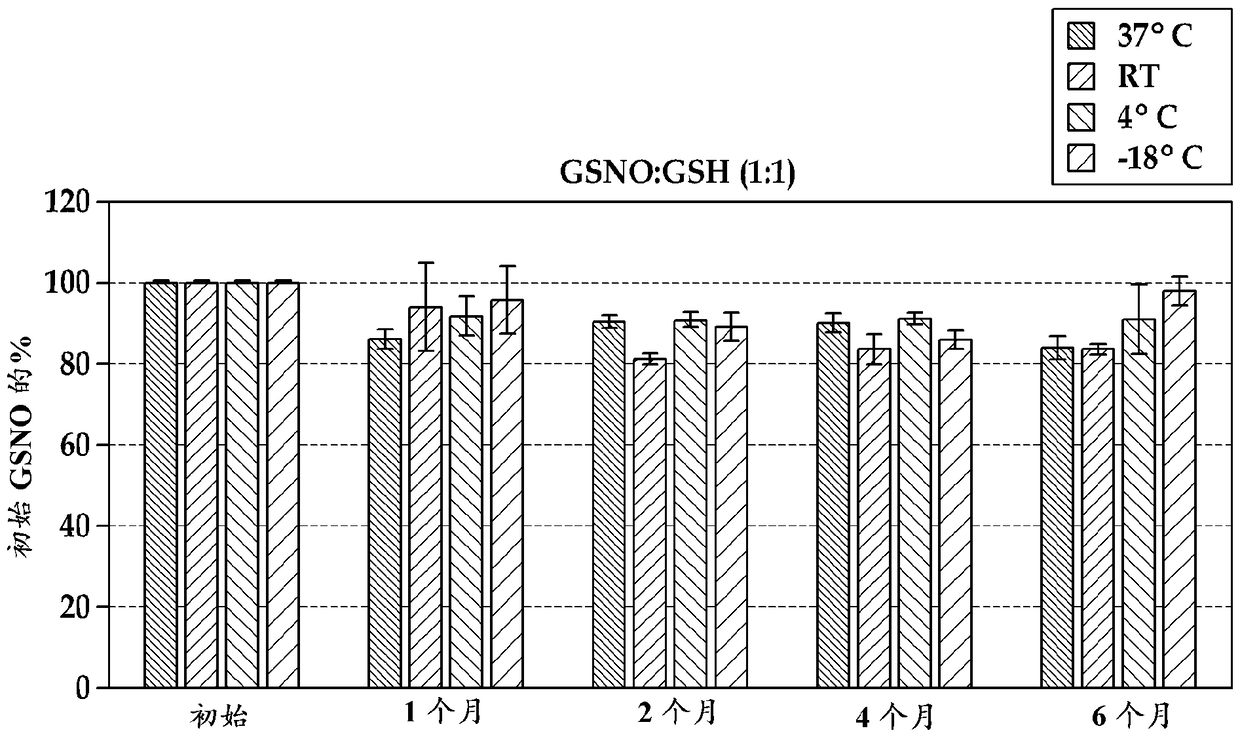
[0079] In this example, using previously synthesized S-nitrosoglutathione (GSNO) alone or in combination with reduced L-glutathione (GSH, >98%) or L-ascorbic acid (Asc), prepared Several nasal preparations. Some formulations also include ethylenediaminetetraacetic acid (EDTA) and / or saline salts (NaCl and NaHCO 3 ). Components were mixed as dry powders and stored in 4 mL amber vials in the freezer (-18°C), at 4°C, at room temperature (RT, about 20°C to about 25°C), or at 37°C.
[0080] Different dry powder nasal formulations include GSNO; GSNO and GSH (1:1 molar ratio); GSNO, GSH and EDTA (1:1:0.1 molar ratio); GSNO:Asc (1:1 molar ratio); and GSNO:Asc :EDTA (1:1:0.1 molar ratio).
[0081] Dry powder nasal formulations of GSNO and EDTA were prepared with saline salt, and dry powder nasal formulations of GSNO and GSH were prepared with saline salt. Both formulations were prepared so that when diluted to 200 mL (with water), the final concentration of ...
Embodiment 3
[0113] 3A – Effect of GSNO:Asc(1:1) on Staphylococcus aureus biofilm disruption
[0114] Three strains of Staphylococcus aureus (SA817, SA1691 and SA 1681) were inoculated in 96-well microtiter plates (1×10 4 colony forming units / 100µl or CFU / 100µl) and cultured overnight. Remove planktonic bacteria and wash once with saline. Different concentrations of GSNO:ascorbic acid (1:1 or equimolar) were diluted in saline, and the saline solution (0.12M, 100 µl) was added to the bacterial biofilm retained on the well surface. Bacteria were incubated at room temperature (RT) for 4 hours, then biofilm density was determined by staining with crystal violet. Each bacterial strain was repeated 6 times, and experiments were carried out 3 times (i.e., for Figure 15 Each data point in N=6, plus or minus standard deviation). The experimental results are shown in Figure 15 middle. Specifically, Figure 15 The effect of GSNO / Asc on 3 different S. aureus isolates (SA817, SA1691 and SA 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com