Oil-phase liquid crystal gel precursor preparation for hydrophilic drug and preparation method of oil-phase liquid crystal gel precursor preparation
A hydrophilic drug and oil phase technology, applied in liquid delivery, drug delivery, pharmaceutical formulations, etc., can solve the problems of poor sustained release effect, poor storage stability, poor stability, etc., to improve bioavailability and solubility. , to maintain the effect of effective concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
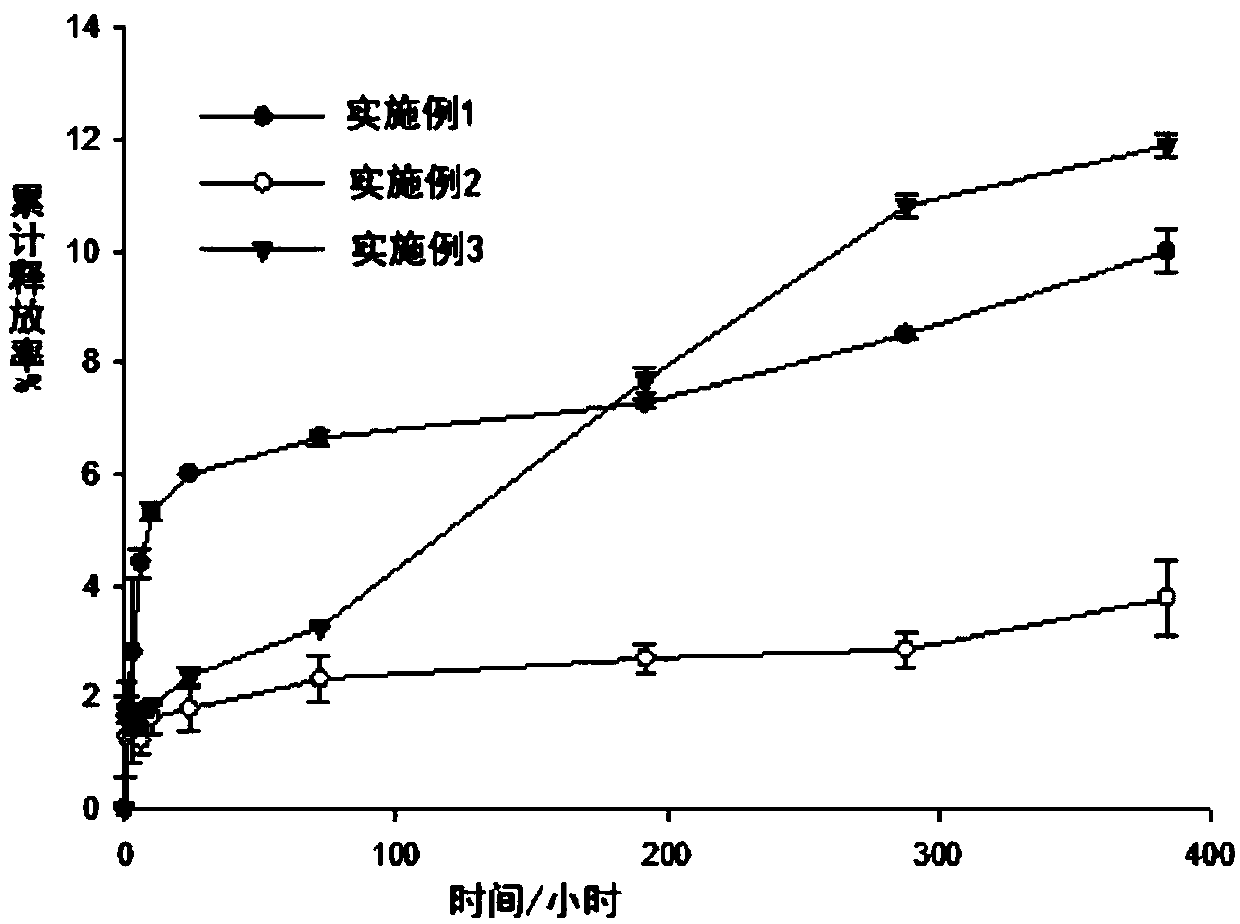
Examples
Embodiment 1
[0032] The oil phase liquid crystal gel precursor preparation of the hydrophilic drug of this embodiment is prepared by the following steps of preparation method:
[0033] (S1) Preparation of phospholipid dispersion: Weigh 1 g of phosphatidylglycerol and 4 g of distilled water into the bottle, seal it, and disperse it on a shaker to form a coarse dispersion; ultrasonically disperse the coarse dispersion for 20 minutes in an ice bath, 50% ( 650W), 1s / 3s, forming a milky white phospholipid dispersion;
[0034] (S2) Preparation of hydrophilic drug solution: Weigh protein PD-15mg and add 1.5ml of water to completely dissolve it to make a 3.33mg / ml drug solution for later use;
[0035] (S3)) Preparation of the water phase: take 140uL of the phospholipid dispersion prepared in the step (S1) and mix with 300uL of the drug solution prepared in the step (S2) to obtain the water phase;
[0036] (S4) Preparation of the oil phase: add 0.3 mg of Tween 80 to 2.7 g of glyceryl dioleate, and...
Embodiment 2
[0045] The oil phase liquid crystal gel precursor preparation of the hydrophilic drug of this embodiment is prepared by the following steps of preparation method:
[0046] (S1) Preparation of phospholipid dispersion: Weigh 1 g of soybean lecithin and 4 g of distilled water into the bottle, seal it, and disperse it on a shaker to form a coarse dispersion; ultrasonically disperse the coarse dispersion for 20 minutes in an ice bath, 50% (650W ), 1s / 3s, forming a milky white phospholipid dispersion;
[0047] (S2) Preparation of hydrophilic drug solution: take 5 mg of protein IgG and add 1.5 ml of water to completely dissolve it to make a 3.33 mg / ml drug solution for subsequent use;
[0048] (S3)) Preparation of the water phase: take 200uL of the phospholipid dispersion prepared in the step (S1) and mix with 300uL of the drug solution prepared in the step (S2) to obtain the water phase;
[0049] (S4) Preparation of the oil phase: add 0.3 mg of Tween 80 to 2.7 g of glyceryl dioleat...
Embodiment 3
[0057] The oil phase liquid crystal gel precursor preparation of the hydrophilic drug of this embodiment is prepared by the following steps of preparation method:
[0058] (S1) Preparation of phospholipid dispersion: Weigh 1g of soybean lecithin, 0.3g of phosphatidylethanolamine and 4g of distilled water into the bottle, seal, and disperse on a shaker to form a coarse dispersion; ultrasonically disperse the coarse dispersion with a probe in an ice bath 20min, 50% (650W), 1s / 3s, forming a milky white phospholipid dispersion;
[0059] (S2) Preparation of hydrophilic drug solution: Weigh 20 mg of insulin and add 1 ml of water to completely dissolve it to make a 20 mg / ml drug solution for later use;
[0060] (S3)) Preparation of the water phase: take 210uL of the phospholipid dispersion prepared in the step (S1) and mix with 300uL of the drug solution prepared in the step (S2) to obtain the water phase;
[0061] (S4) Preparation of the oil phase: add 1 mg of Tween 80 to 2.7 g of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com