Zinc borate 2335 preparation method and zinc borate 2335 particle diameter control method
A zinc borate, particle size technology, applied in borates, chemical instruments and methods, boron compounds, etc., can solve the problems of uneven product size distribution, irregular shape, single reaction conditions, etc., to avoid surface crystallization Effect of Incomplete or Severe Agglomeration Phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
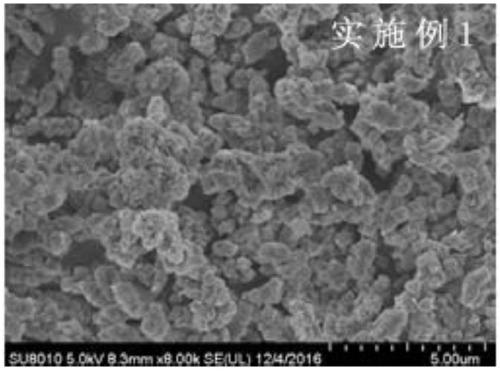
Embodiment 1
[0037] Prepare 45g of zinc sulfate into a solution with a content of 35%, add it into the reaction kettle, start stirring, mix 67g of borax, 0.3g of zinc oxide, 15g of boric acid, 15g of sodium sulfate, 0.03g of 0.5-3μm Zinc borate crystal seeds are added to the reactor, and a certain amount of water is added to make the system solid-liquid ratio 1:1. The temperature is raised to 97°C for reaction, and the reaction is kept for 5 hours. After the reaction, it takes 75 minutes to add 75 parts of 82°C water to the reactor. , reduce the motor speed, and the system will naturally cool down to 60°C. The reaction solution is centrifuged, washed, and the filter cake is air-dried to obtain the product of the present invention.
Embodiment 2
[0039] Prepare 40g of zinc sulfate into a solution with a content of 35%, add it into the reaction kettle, start stirring, mix 70g of borax, 0.5g of zinc oxide, 2g of boric acid, 2g of sodium sulfate, 0.03g of 6-10μm Zinc borate crystal seeds are added to the reactor, and a certain amount of water is added to make the system solid-liquid ratio 1:1, and the temperature is raised to 97°C for reaction, and the reaction is kept for 5 hours. After the reaction, it takes 120 minutes to add 100 parts of 95°C water to the reactor , reduce the motor speed, and the system will naturally cool down to 60°C. The reaction solution is centrifuged, washed, and the filter cake is air-dried to obtain the product of the present invention.
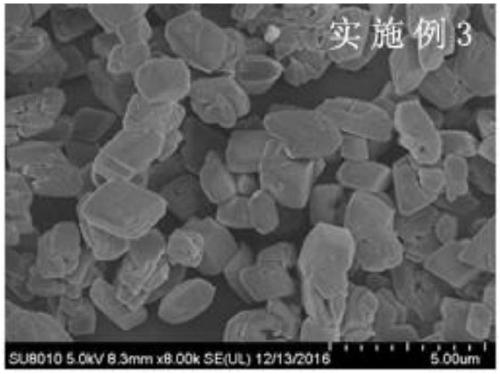
Embodiment 3
[0041]Prepare 50g of zinc sulfate into a solution with a content of 35%, add it into the reaction kettle, start stirring, mix 60g of borax, 0.1g of zinc oxide, 7g of boric acid, 7g of sodium sulfate, 0.03 parts of 3-6μm Zinc borate crystal seeds were added to the reactor, and a certain amount of water was added to make the system solid-to-liquid ratio 1:1. The temperature was raised to 105°C for reaction, and the reaction was kept for 5 hours. After the reaction, it took 30 minutes to add 50g of 70°C water to the reactor. Reduce the motor speed, and the system naturally cools down to 60°C. The reaction solution is centrifuged, washed, and the filter cake is air-dried to obtain the product of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com