Method for generating benzisothiazolinone compounds by catalyzing oxidization of molecular oxygen in aqueous phase
A technology of benzisothiazolinone and compound, which is applied in the field of organic sulfur-nitrogen bond synthesis, can solve the problems of generating toxic waste, long reaction time, excessive additives, etc., and achieves less by-products, high reaction efficiency and environmental friendliness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation method of the benzisothiazolinone compound A of the following structural formula:
[0029]
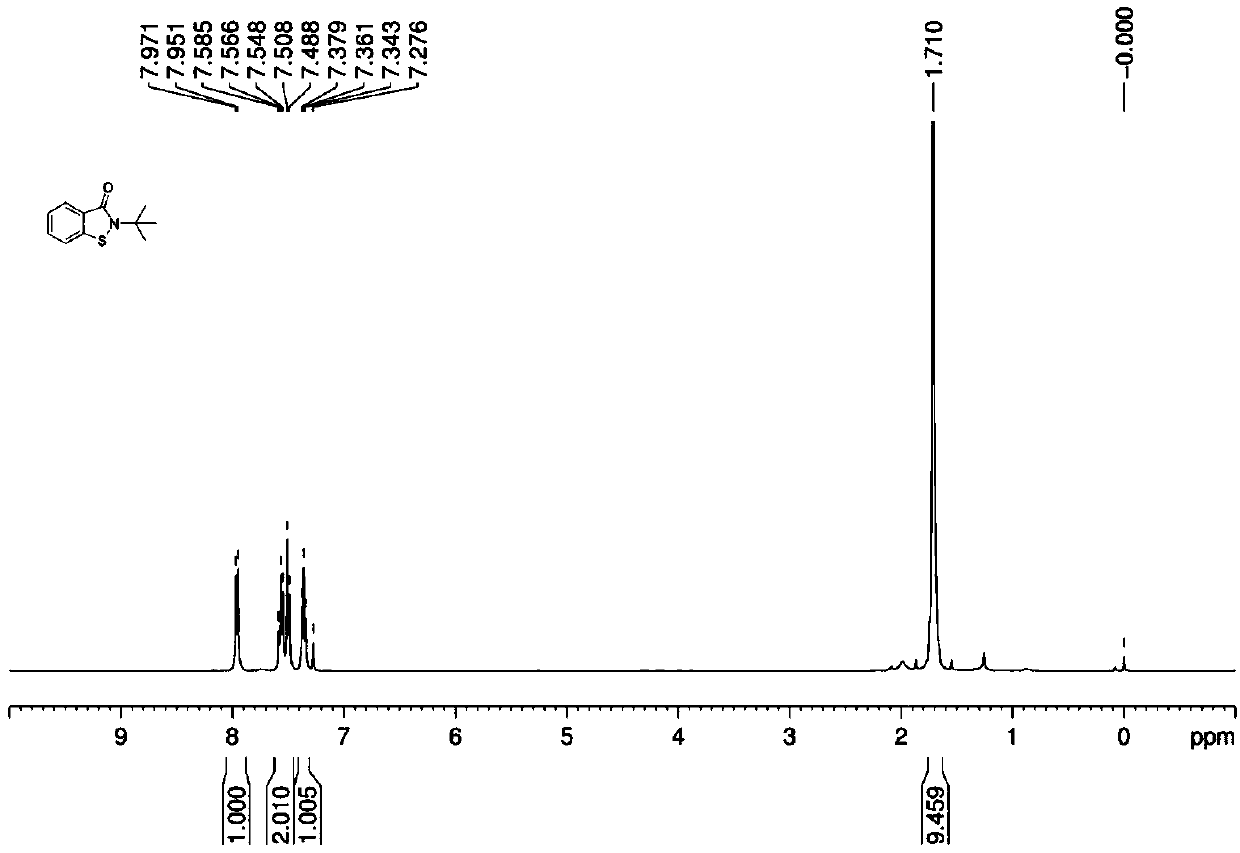
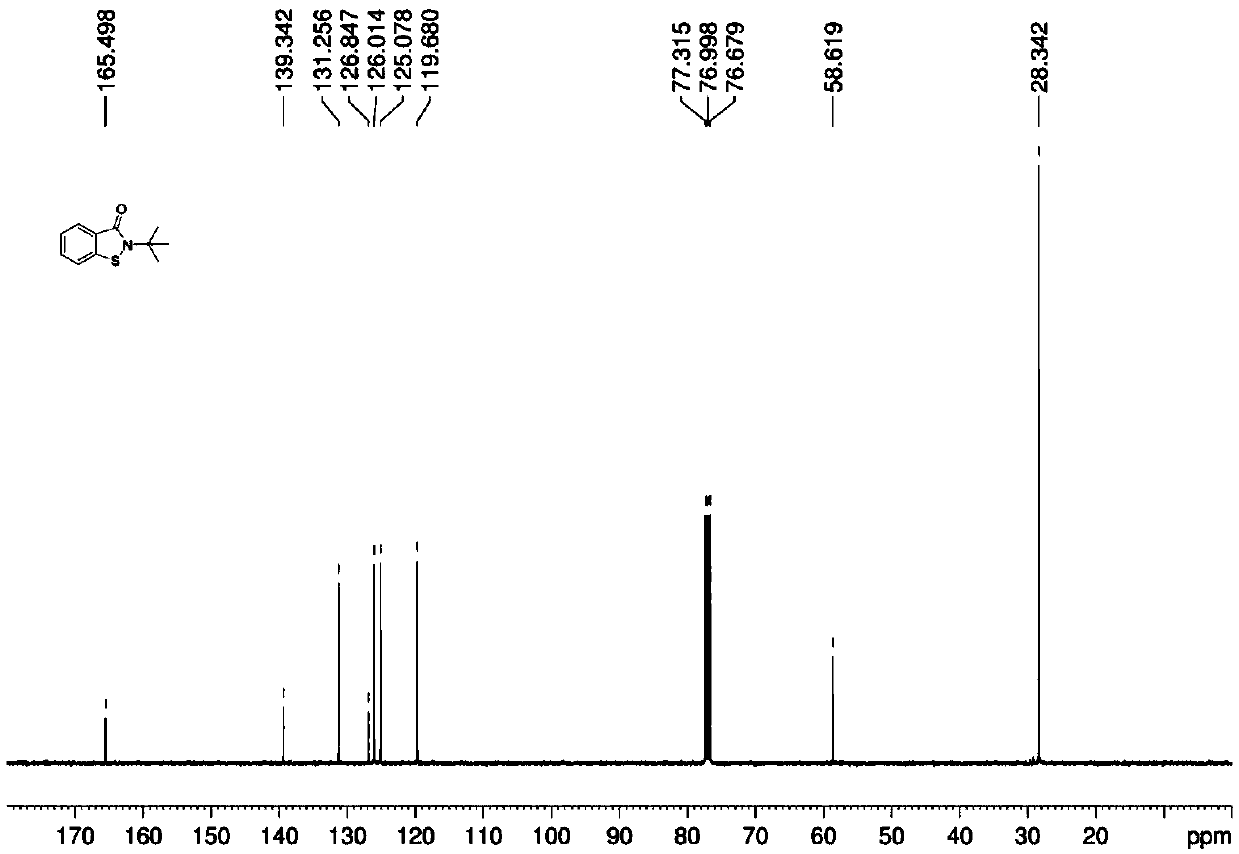
[0030] In the 50mL flask, add 2g N-tert-butyl thiosalicylic acid amide, 0.1g (5wt%) Tetrasodium iron tetracarboxyphthalocyanine [FePc(CO 2 Na) 4 ], 0.02g (1wt%) tetracarboxy manganese phthalocyanine tetrasodium [MnPc(CO 2 Na) 4 ] and 30mL of water, poured into oxygen, heated to 100°C, and reacted for 2 hours. After the reaction, cooled, filtered, washed the filter cake with 20mL of water, and dried to obtain 1.9 g of white solid. The structure of the product was determined by NMR, MS and other methods as the target The yield of product A is 96%, and the purity of the product by liquid chromatography analysis is 99%.
[0031] 1 H NMR (600MHz, CDCl 3 ,SiMe 4 )δ: 7.96 (d, J = 7.8Hz, 1H), 7.59-7.49 (m, 2H), 7.36 (t, J = 7.4Hz, 1H), 1.71 (s, 9H);
[0032] 13 C NMR (150MHz, CDCl 3 ,SiMe 4 )δ: 165.5, 139.3, 131.3, 126.8, 126.0, 125.1, 119.7, 58.6, 28.3.
Embodiment 2
[0034] The preparation method of the benzisothiazolinone compound B of the following structural formula:
[0035]
[0036] In the 100mL flask, add 4g N-phenyl thiosalicylic acid amide, 0.08g cobalt tetrasodium tetrasulfonate phthalocyanine [CoPc(SO 3 Na) 4 ], 0.08g copper tetracarboxyphthalocyanine [CuPc(CO 2 h)4 ], 0.12g tetrasulfonic manganese phthalocyanine [MnPc(SO 3 h) 4 ] and 50mL water, poured into oxygen, heated to 80°C, reacted for 12h, after the reaction was finished, cooled, filtered, washed the filter cake with 40mL water, dried to obtain 3.1g target product B, the yield was 78%, liquid chromatography analysis The product purity is 99%.
Embodiment 3
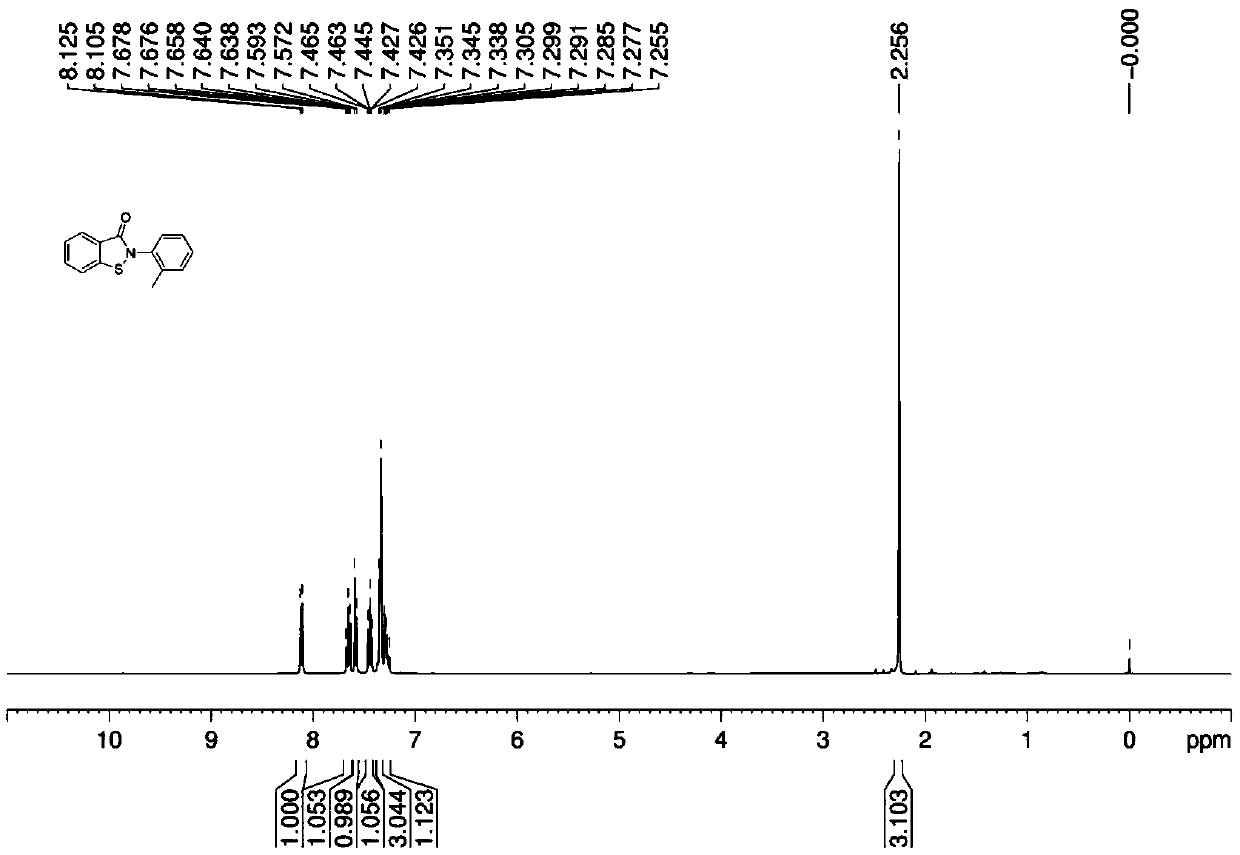
[0038] The preparation method of the benzisothiazolinone compound C with the following structural formula:
[0039]
[0040] In the 250mL flask, add 10g N-(2-methyl) phenyl thiosalicylic acid amide, 0.1g tetrapotassium tetrasulfonic manganese phthalocyanine [MnPc(SO 3 K) 4 ] and 100mL water, poured into oxygen, heated to 90°C, reacted for 24h, after the reaction was finished, cooled, filtered, washed the filter cake with water, dried to obtain 8.2g target product C, the yield was 82%, and the product was analyzed by liquid chromatography The purity is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com