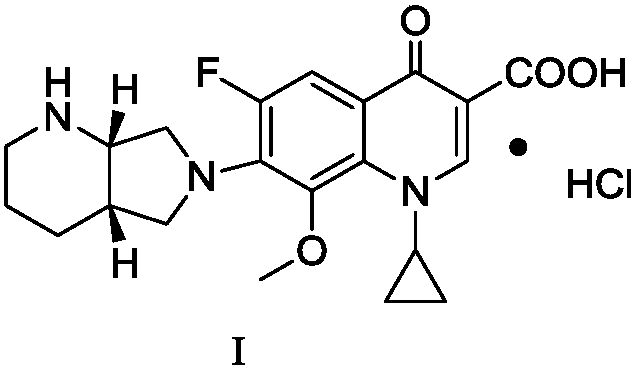
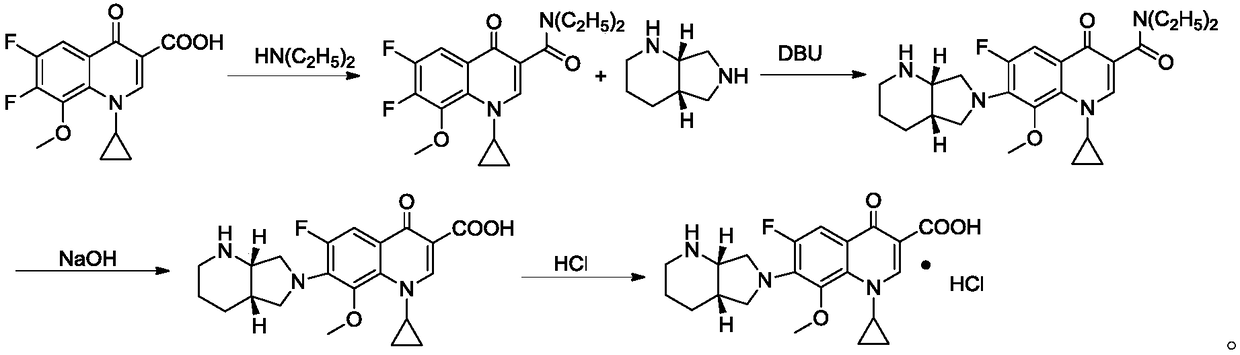
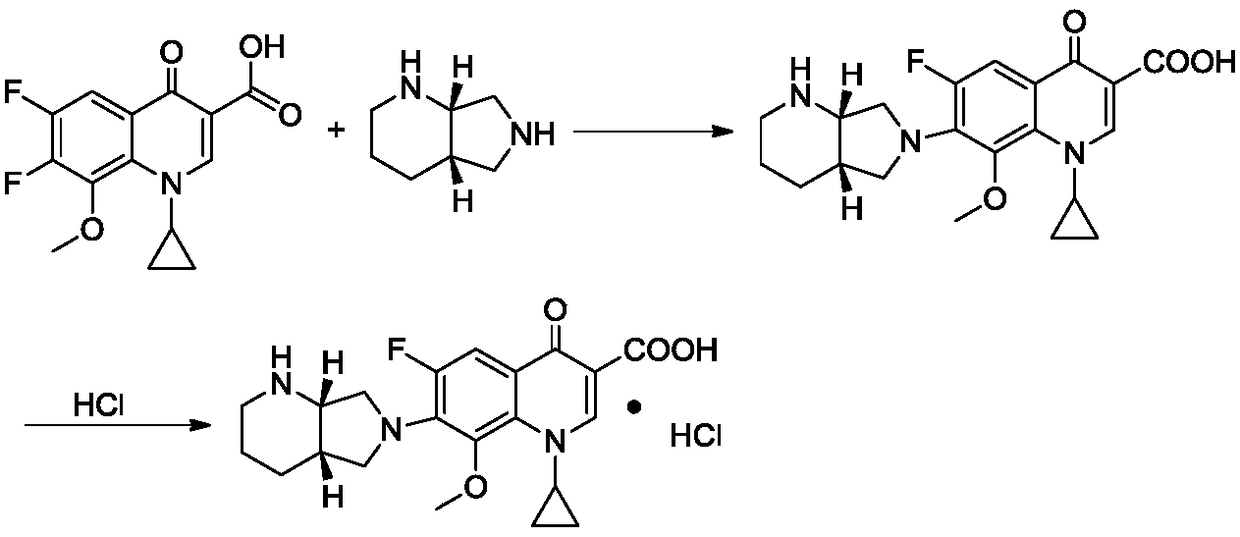
Preparation method of moxifloxacin hydrochloride and intermediate thereof
A technology for moxifloxacin hydrochloride and intermediates is applied in the field of preparation of moxifloxacin hydrochloride and intermediates thereof, and can solve the problems of unsuitability for industrial production, poor product purity and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the preparation of moxifloxacin hydrochloride intermediate II
[0064]
[0065] Under nitrogen protection, 10.0Kg (33.87 mol) and 7.95Kg (89.19mol) of 2-amino-2-methyl-1-propanol, heated to 105-115°C and refluxed for 6-7 hours and separated the produced water until it reached the measured amount or no longer increased . Cool to 10 ℃~20 ℃, add mass concentration successively respectively and be 2% acetic acid aqueous solution (the described mass concentration refers to the percentage that the quality of acetic acid accounts for the total mass of the acetic acid aqueous solution), and the mass concentration is 5% sodium carbonate aqueous solution (the described Mass concentration refers to the quality of sodium carbonate accounts for the percentage of the total mass of sodium carbonate aqueous solution), mass concentration is 7% sodium bicarbonate aqueous solution (described mass concentration refers to the quality of sodium bicarbonate accounts for the ...
Embodiment 2
[0066] Embodiment 2: the preparation of moxifloxacin hydrochloride intermediate II
[0067]Under nitrogen protection, 50.0 g (0.169 mol) and 60.4 g (0.678 mol) of 2-amino-2-methyl-1-propanol, heated to 75-85° C. and refluxed for 12-14 hours and separated the produced water. Cool to 10 ℃~20 ℃, add mass concentration successively respectively and be 2% acetic acid aqueous solution (the described mass concentration refers to the percentage that the quality of acetic acid accounts for the total mass of the acetic acid aqueous solution), and the mass concentration is 5% sodium carbonate aqueous solution (the described Mass concentration refers to the quality of sodium carbonate accounts for the percentage of the total mass of sodium carbonate aqueous solution), mass concentration is 7% sodium bicarbonate aqueous solution (described mass concentration refers to the quality of sodium bicarbonate accounts for the percent of the total mass of sodium bicarbonate aqueous solution) , mas...
Embodiment 3
[0068] Embodiment 3: the preparation of moxifloxacin hydrochloride intermediate II
[0069] Under nitrogen protection, 20.0 g of 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid was added to 150 mL of ethylbenzene ( 0.0677mol) and 10.9g (0.122mol) of 2-amino-2-methyl-1-propanol, heated to 130°C to 140°C and refluxed for 3 hours to 4 hours and separated the produced water. Cool to 10 ℃~20 ℃, add mass concentration successively respectively and be 2% acetic acid aqueous solution (the described mass concentration refers to the percentage that the quality of acetic acid accounts for the total mass of the acetic acid aqueous solution), and the mass concentration is 5% sodium carbonate aqueous solution (the described Mass concentration refers to the quality of sodium carbonate accounts for the percentage of the total mass of sodium carbonate aqueous solution), mass concentration is 7% sodium bicarbonate aqueous solution (described mass concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com