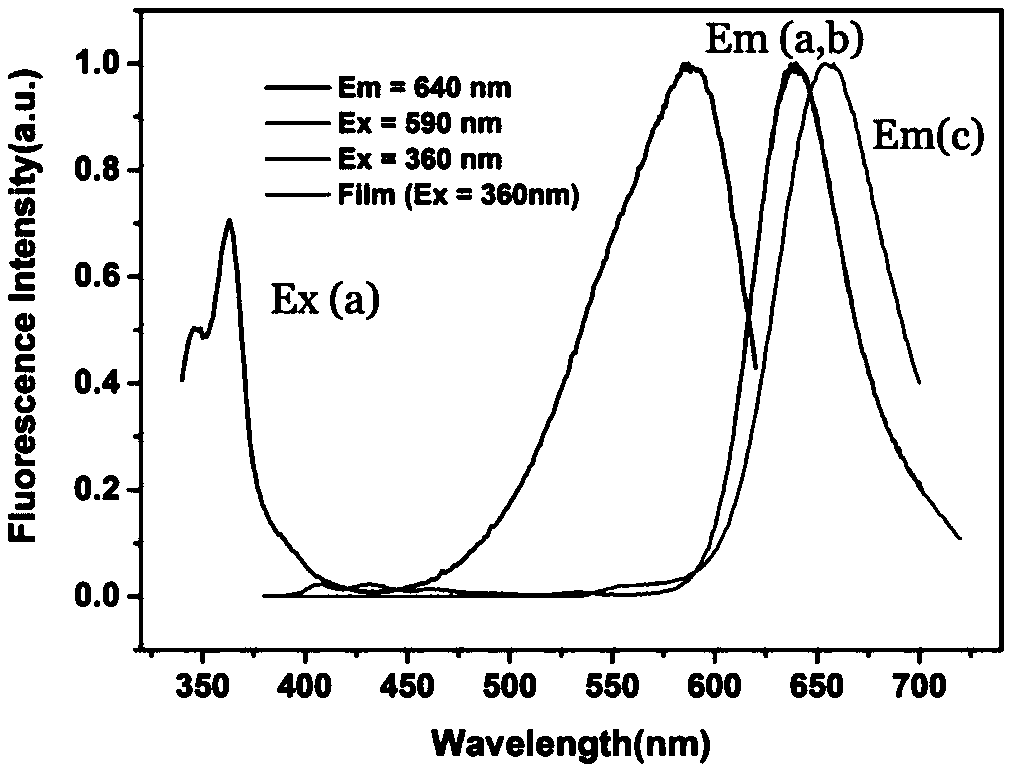
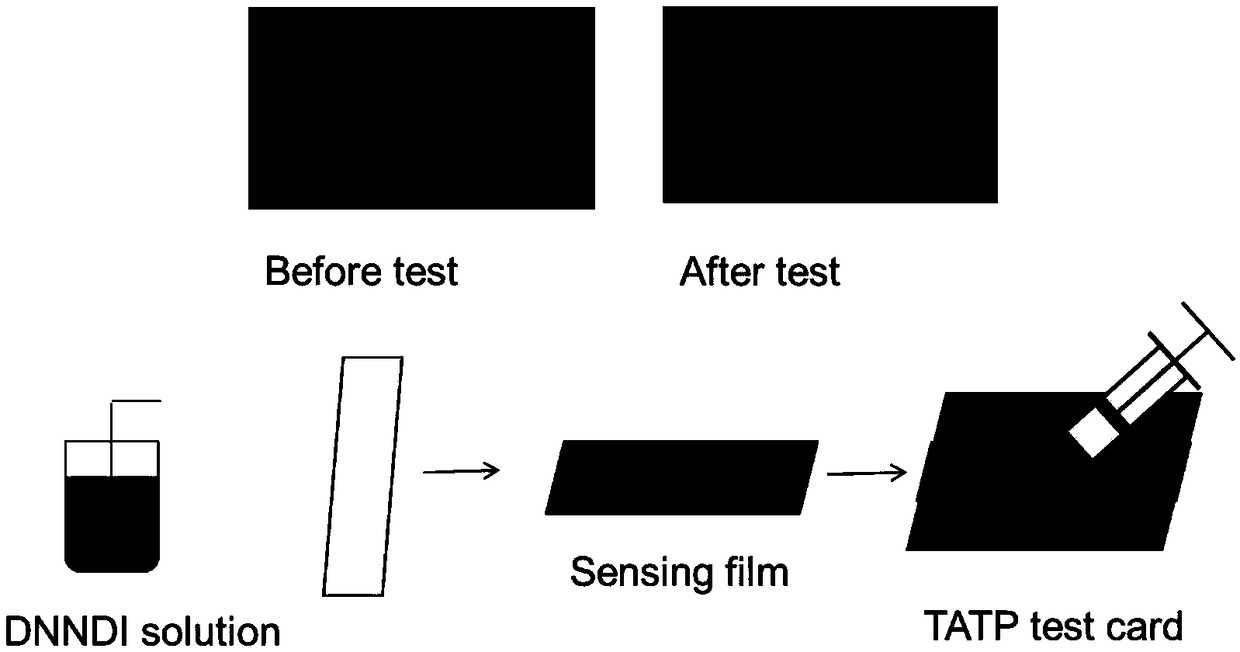
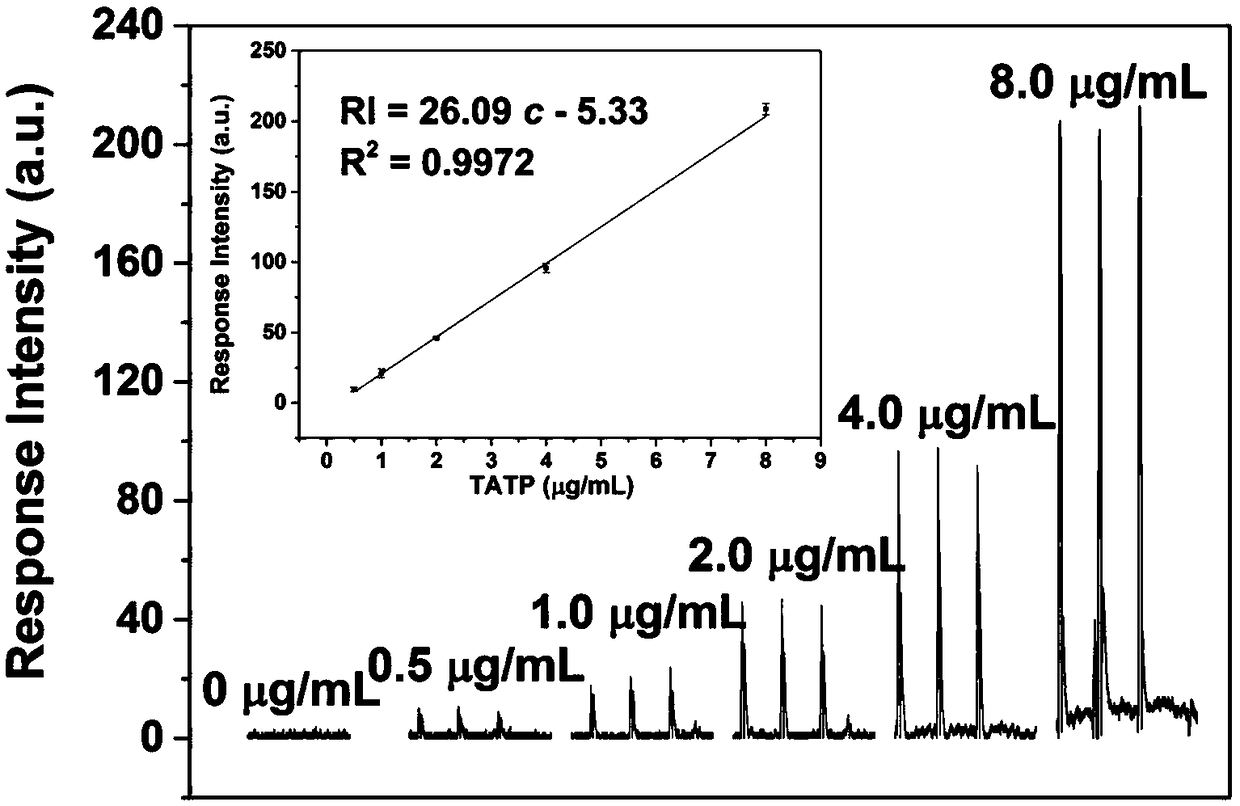
Fluorescent chemical compound for TATP visual detection, preparation method and application thereof
A fluorescent compound and compound technology, applied in the field of fluorescent compounds for TATP visual detection and its preparation, can solve the problems of sensitivity, selectivity, and intuitiveness that are difficult to meet actual needs, and there are no examples of reversible multiple TATP visual detection, etc., to achieve Stable and reliable sensing response signal, beneficial to capture and adsorption, good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1) Under the protection of argon, dissolve 2.5g of 1,5-dibromo-2,3,6,7-naphthalene tetracarboxylic dianhydride in 40mL of acetic acid solution, stir thoroughly for 30min, then add 2.45mL of R', React at 120°C for 4 hours. After the reaction is complete, pour the reaction solution into ice water, and solids can be seen to precipitate out. After stirring for 1 hour, suction filter, wash with water and ethanol several times, and freeze-dry the obtained filter cake. The solid was purified by silica gel column chromatography, using a mixture of dichloromethane: acetone = 100:1 as the eluent to obtain a light yellow solid which is compound I;
[0053] Among them, the molar ratio of 1,5-dibromo-2,3,6,7-naphthalene tetracarboxylic dianhydride to R' and acetic acid is 1:3:119, and the structure of R' is n=5.
[0054] 2) Under anhydrous and oxygen-free conditions, weigh 0.5g of compound I, catalyst Ruphos 0.045g, RuPhosPd G30.061g, and CsCO 3 Put 0.718g in a two-necked flask,...
Embodiment 2
[0060] 1) Under the protection of argon, dissolve 2.5g of 1,5-dibromo-2,3,6,7-naphthalene tetracarboxylic dianhydride in 40mL of acetic acid solution, stir thoroughly for 30min, then add 2.85mL of R', React at 120°C for 4 hours. After the reaction is complete, pour the reaction solution into ice water, and solids can be seen to precipitate out. After stirring for 1 hour, suction filter, wash with water and ethanol several times, and freeze-dry the obtained filter cake. The solid was purified by silica gel column chromatography, using a mixture of dichloromethane: acetone = 100:1 as the eluent to obtain a light yellow solid which is compound I;
[0061] Among them, the molar ratio of 1,5-dibromo-2,3,6,7-naphthalene tetracarboxylic dianhydride to R' and acetic acid is 1:3:119, and the structure of R' is n=8.
[0062] 2) Under anhydrous and oxygen-free conditions, weigh 0.5g of compound I, 0.031g of catalyst Ruphos, 0.055g of RuPhosPd G3, and CsCO 3 Put 0.639g in a two-necked...
Embodiment 3
[0065] 1) Under the protection of argon, dissolve 2.5g of 1,5-dibromo-2,3,6,7-naphthalene tetracarboxylic dianhydride in 40mL of acetic acid solution, stir thoroughly for 30min, then add 3.5g of R', React at 120°C for 4 hours. After the reaction is complete, pour the reaction solution into ice water, and solids can be seen to precipitate out. After stirring for 1 hour, suction filter, wash with water and ethanol several times, and freeze-dry the obtained filter cake. The solid was purified by silica gel column chromatography, using a mixture of dichloromethane: acetone = 100:1 as the eluent to obtain a light yellow solid which is compound I;
[0066] Among them, the molar ratio of 1,5-dibromo-2,3,6,7-naphthalene tetracarboxylic dianhydride to R' and acetic acid is 1:3:119, and the structure of R' is n=12.
[0067] 2) Under anhydrous and oxygen-free conditions, weigh 0.5g of compound I, 0.027g of catalyst Ruphos, 0.048g of RuPhosPd G3, and CsCO 3 Put 0.557g in a two-necked ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com