Derivative containing salicylic acid harringtonine, production method and uses thereof
A technology containing harringtonine salicylate and harringtonine salicylate, which is applied in the field of derivatives containing harringtonine salicylate, and can solve the problems of fast metabolism, limited application, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
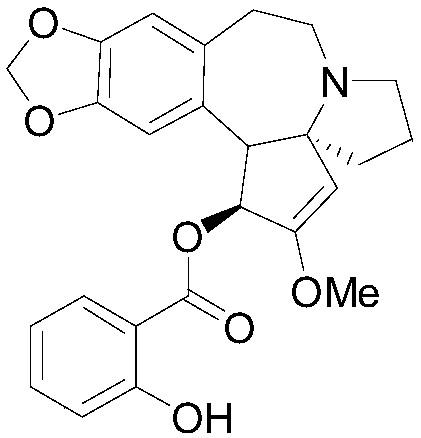
[0090] The synthesis of embodiment 1 compound 01
[0091] (1S,3aR)-2-Methoxy-1,5,6,8,9,14b-octahydro-4H-[1,3]dioxole[4',5':4, 5] Benzo[1,2-d]cyclopentadiene[b]pyrrolo[1,2-a]azepin-1-yl 2-hydroxybenzoate (compound 01)
[0092] React 0.02mol salicylic acid with 4ml of thionyl chloride at 70°C, add 4 drops of catalyst pyridine, and react for 2 hours to remove excess SOCl2 by suction filtration under reduced pressure, dissolve with 10ml of dichloromethane, and stir in an ice bath . 0.01mol harringtonine was dissolved in anhydrous chloroform, and then 0.02mol DDC and 0.002molPP were dissolved in anhydrous chloroform, added to the reaction solution under nitrogen protection, and stirred at room temperature for three days. The target product was obtained by column chromatography with ethyl acetate:petroleum ether=1:3. The relevant data are as follows:
[0093] Compound 01EI-MS m / z:435.4; Anal.Calcd.For C25H25NO6:C,68.95;H,5.79;N,3.22;Found C,68.97;H,5.81;N,3.24
Embodiment 2
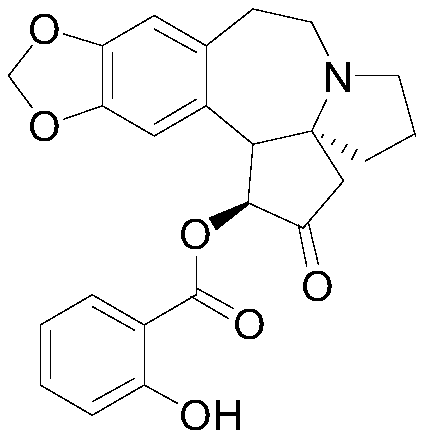
[0094] The synthesis of embodiment 2 compound 02
[0095] (1S,3aS)-2-Oxo-1,2,3,5,6,8,9,14b-octahydro-4H-[1,3]dioxole[4',5': 4,5]Benzo[1,2-d]cyclopentadiene[b]pyrrolo[1,2-a]azepin-1-yl 2-hydroxybenzoate (compound 02)
[0096] With 0.01mol(1S,3aS)-1-hydroxyl-1,5,6,8,9,14b-hexahydro-4H-[1,3]dioxole[4',5':4 ,5] Benzo[1,2-d]cyclopentadiene[b]pyrrolo[1,2-a]azepine-2(3H)-one instead of harringtonine can obtain the target according to the operation of Example 1 product. The relevant data are as follows:
[0097] Compound 02EI-MS m / z:421.1; Anal.Calcd.For C24H23NO6:C,68.40;H,5.50;N,3.32;Found C,68.42;H,5.52;N,3.30
Embodiment 3
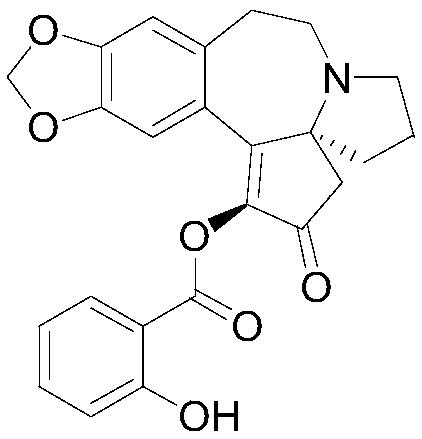
[0098] The synthesis of embodiment 3 compound 03
[0099] (S)-2-Oxo-2,3,5,6,8,9-hexahydro-4H-[1,3]dioxole[4',5':4,5]benzene And[1,2-d]cyclopentadiene[b]pyrrolo[1,2-a]azepin-1-yl 2-hydroxybenzoate (compound 03)
[0100] With 0.01mol(S)-1-hydroxy-5,6,8,9-tetrahydro-4H-[1,3]dioxole[4',5':4,5]benzo[ 1,2-d]cyclopentadiene[b]pyrrolo[1,2-a]azepin-2(3H)-one instead of harringtonine The target product can be obtained according to the operation of Example 1. The relevant data are as follows:
[0101] Compound 03EI-MS m / z:419.4; Anal.Calcd.For C24H21NO6:C,68.73;H,5.05;N,3.34;Found C,68.71;H,5.07;N,3.36
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com