Selenium-containing compound and use thereof
A compound and application technology, applied in the field of selenium-containing compounds and their preparation, can solve problems such as side effects, limited anti-cancer spectrum, and limited compound structure types, and achieve the effects of simple preparation method, high yield, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
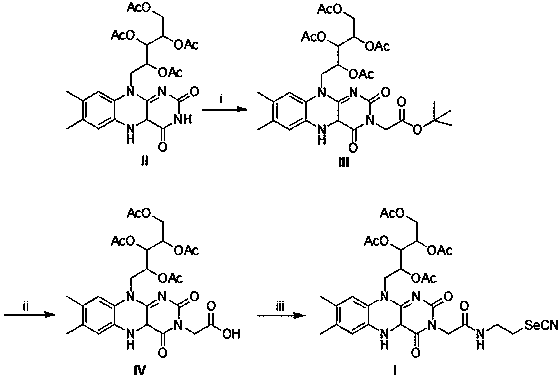
[0043] Embodiment 1: the synthesis of formula II compound
[0044] In a three-neck flask, dissolve riboflavin (1 g, 2.66 mmol) in pyridine (10 mL), add acetic anhydride (1.356 g, 13.3 mmol), stir for 16 hours, and TLC detects that the reaction is complete. The reaction solution was dissolved in 30 mL of water, the aqueous phase was extracted with dichloromethane (10 mL×3), the combined organic phase was washed with saturated brine (20 mL×1), and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the obtained crude product was purified by column chromatography (ethyl acetate:petroleum ether (v / v)=20:1) to obtain 721 mg of a yellow powdery solid with a yield of 50%.
[0045] nuclear magnetic resonance 1 H NMR (400 MHz, CDCl 3 ) δ:8.60(s, 1H), 8.03(s, 1H), 7.57(s,1H), 5.68~5.66(d, 1H), 6.46~5.39(m,2H), 4.91(s, 1H), 4.45~4.42(m, 1H), 4.27~4.22(m, 1H), 2.57(s, 3H), 2.45(s, 3H), 2.30(s, 3H), 2.22(s, 3H), 2.08(s, 3H), 1.76(s, 4H).
[0...
Embodiment 2
[0047] Embodiment 2: the synthesis of formula III compound
[0048] In a three-hole flask, dissolve compound II (670 mg) in anhydrous DMF (5 mL), add potassium carbonate (204 mg), potassium iodide (20 mg), stir for 30 minutes, and then add tert-butyl bromoacetate (1.68 g), after addition, reaction solution is warming up to 40 DEG C, continues reaction 16 hours. TLC showed that the reaction was complete. After the reaction solution was cooled to room temperature, water (10 mL) was added, the aqueous phase was extracted with ethyl acetate (15 mL×3), and the combined organic phase was washed with saturated brine (20 mL×1). water and sodium sulfate to dry. The solvent was distilled off under reduced pressure, and the obtained crude product was purified by column chromatography (ethyl acetate:petroleum ether=5:1) to obtain a yellow powdery solid (770 mg), with a yield of 95%.
[0049] nuclear magnetic resonance 1 H NMR (400 MHz, CDCl 3 ) δ:8.02(s, 1H), 7.55(s, 1H), 5.66~5.64(d,...
Embodiment 3
[0051] Embodiment 3: the synthesis of formula IV compound
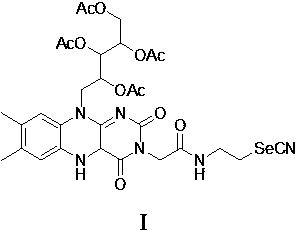
[0052] Compound III (200 mg, 0.3 mmol) and 1 mL of anhydrous dichloromethane were added to a 100 ml three-necked flask, and the mixture was cooled to 0°C under protection of argon, and 0.3 mL of trifluoroacetic acid was added, heated to 50°C and stirred for 5 h. After the reaction is over, pour the reaction solution into ice water, adjust the pH to 5 with saturated sodium bicarbonate solution, add dichloromethane for extraction, collect the organic phase and wash it with saturated brine, then with water, and dry the collected organic phase with anhydrous magnesium sulfate , filtered with suction, dried in vacuo, and evaporated to dryness. Column chromatography (ethyl acetate: ethanol = 1:1 (V:V)) gave 120 mg of a light yellow solid (compound IV), yield: 66%.
[0053] nuclear magnetic resonance 1 H NMR (400 MHz, D 2 O) δ: 7.82 (s,1H), 7.74 (s, 1H), 5.67-5.65(m, 1H), 5.53 (br t, J=6, 1H), 5.49-4.45(m, 1H), 5.10 ( br ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| NMR spectroscopy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com