Composition for use in the prophylaxis of allergic disease
A technology for allergic diseases and compositions, which can be used in allergic diseases, sensory diseases, drug combinations, etc., and can solve problems such as expensive and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Materials and methods
[0088] Soybean Oil - Obtained from Florin AG, Switzerland (Florin AG, Switzerland; Lot No. 280813). The fatty acids of the oil are mainly composed of saturated fatty acids palmitic acid (14%) and stearic acid (4%), n-6PUFA LA (47%) and GLA (0.2%), oleic acid (27%) and n-3PUFA ALA (5 %)composition. DGLA, EPA and DHA were undetectable.
[0089] High DHA fish oil (containing 20-26% docosahexaenoic acid (DHA; 22:6n3) and 7% eicosapentaenoic acid (EPA; 20:5n3) was obtained from Sofinol SA (Nestlé corporation ) subsidiary). Other major fatty acids consist of palmitic acid (21%), palmitoleic acid (3%), stearic acid (5%) and oleic acid (19%). GLA and DGLA levels are below 0.2%, The ARA content is about 1%.
[0090] DGLA oil is derived from the fungus Mortierella alpina from Nippon Suisan Kaisha Ltd. The DGLA content is 35.6%. Other major fatty acids consist of palmitic acid (16.3%), stearic acid (7.1%), oleic acid (8.2%), LA (6.4%), GLA (2.6%), b...
Embodiment 2
[0103] Materials and methods
[0104] RBL-2H3 cells; rat basophilic leukemia cells were cultured under standard conditions in a culture medium containing 15% serum. Once a sufficient number of cells were present in the dish, the sodium salts of the various fatty acids were added directly to the medium and the cells were incubated with these fatty acids for 24 hours. Cells also received phorbol 12-myristate 13-acetic acid ("PMA"; final concentration 50 ng / mL) and ionomycin ("IM"; final concentration 0.125 μΜ) during the last 18 hours of this incubation period , to stimulate cells to secrete IL4.
[0105] result
[0106] Figure 5 It is shown that cells incubated with DGLA (final concentration 60 μΜ) produced less IL4 after stimulation with PMA and IM than cells not treated with additional fatty acids (control). The same was observed for cells incubated with 2.14 μM of a fatty acid mixture ("NIF") consisting of DHA and EPA mixed in a 3:1 ratio. A synergistic decrease in...
Embodiment 3
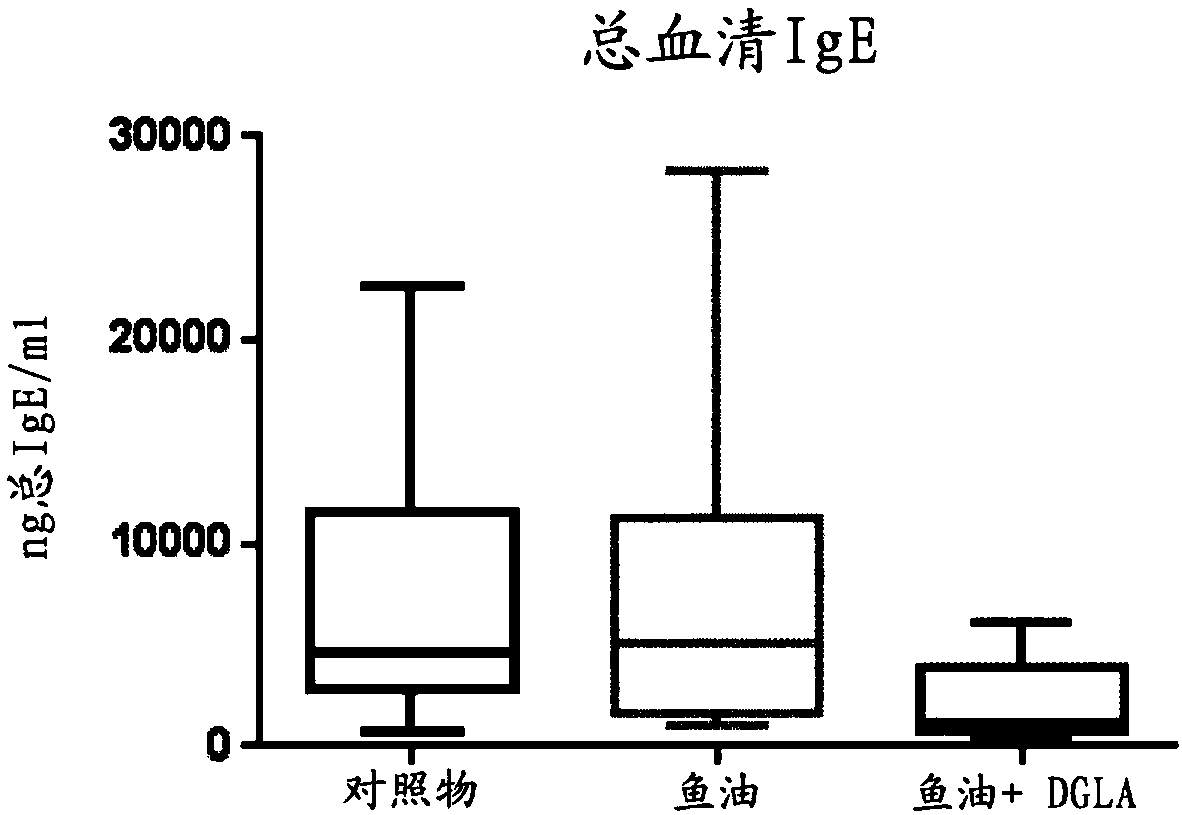
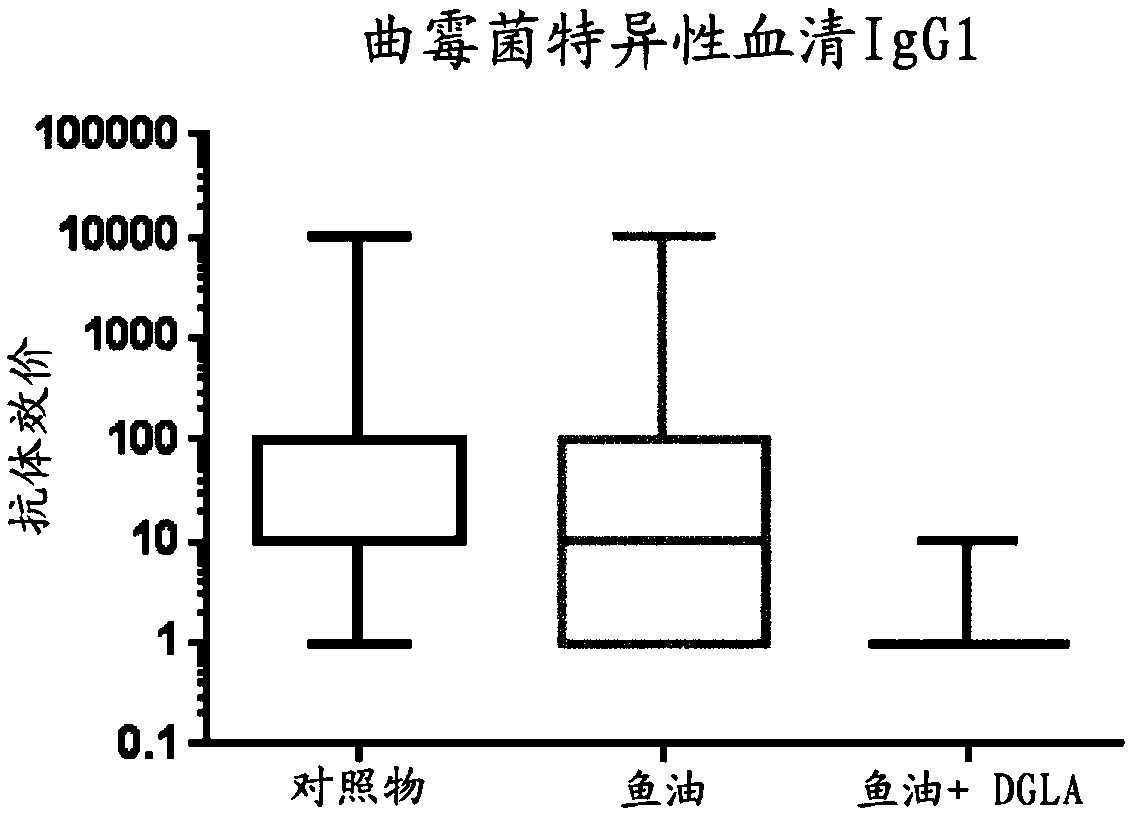
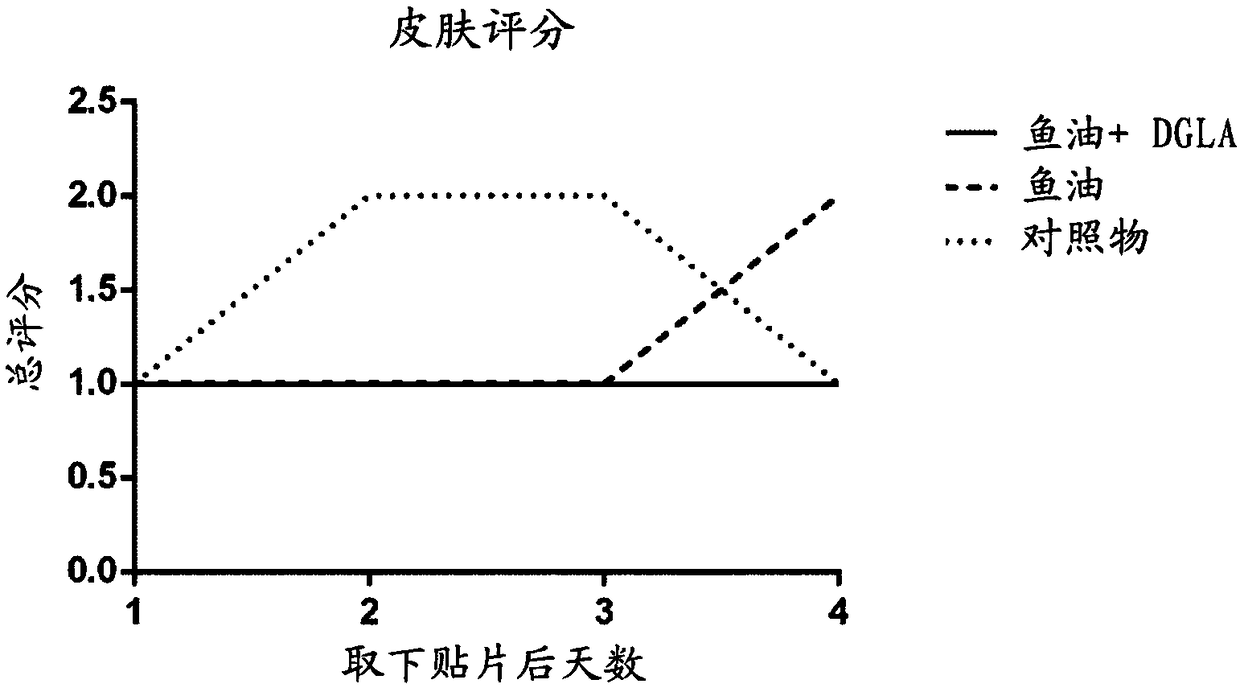
[0108] Pregnant mice were divided into 2 groups and fed a low-fat based diet (standard rodent diet) from gestation day 5 until weaning of the pups, in which diet was added (per 95 g of the diet): 5 g soybean oil ( control group) or 2.5g fish oil+2.5g DGLA oil (fish oil+DGLA group). The oil composition is described in Example 1.
[0109] From the second week of life, the pups were able to chew the food in the cage, so to avoid direct exposure of the pups to the diet, the mother and her pups were fed a control diet, but the mother was given an additional daily via gavage: 0.1ml Soybean oil or 0.05ml high DHA fish oil + 0.05ml DGLA oil. Therefore, the pups never had direct access to the diet.
[0110] After weaning, all pups received a control diet. Two weeks after weaning, pups (10 pups per group) received skin patches as described in Example 1. On the last day of skin assessment, mice were humanely sacrificed and skin-draining brachial lymph nodes were collected.
[0111] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com