Anti-crispr compounds and methods of use
A composition and compound technology, applied in the direction of biochemical equipment and methods, microorganisms, drug combinations, etc., can solve the problems of lack of predictable control and robust inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0346] Construction of Acr vector
[0347] Sequences found to encode the Acr protein can be ordered from companies that sell custom DNA sequence samples, or they can be PCR amplified from appropriate biological DNA samples. They can then be introduced into vectors (including but not limited to plasmid and viral vectors) by standard recombinant DNA cloning protocols (eg, restriction endonuclease digestion and religation, or Gibson assembly). The vector typically includes a promoter sequence suitable for expression in the cell of interest. They also contain other sequences that facilitate gene expression and control [eg, 5' and 3' untranslated regions, poly(A) signals, and transcription terminators]. These vectors can then be introduced into target cells by using standard delivery methods well known in the art.
Embodiment II
[0349] Screening of Acr protein candidates
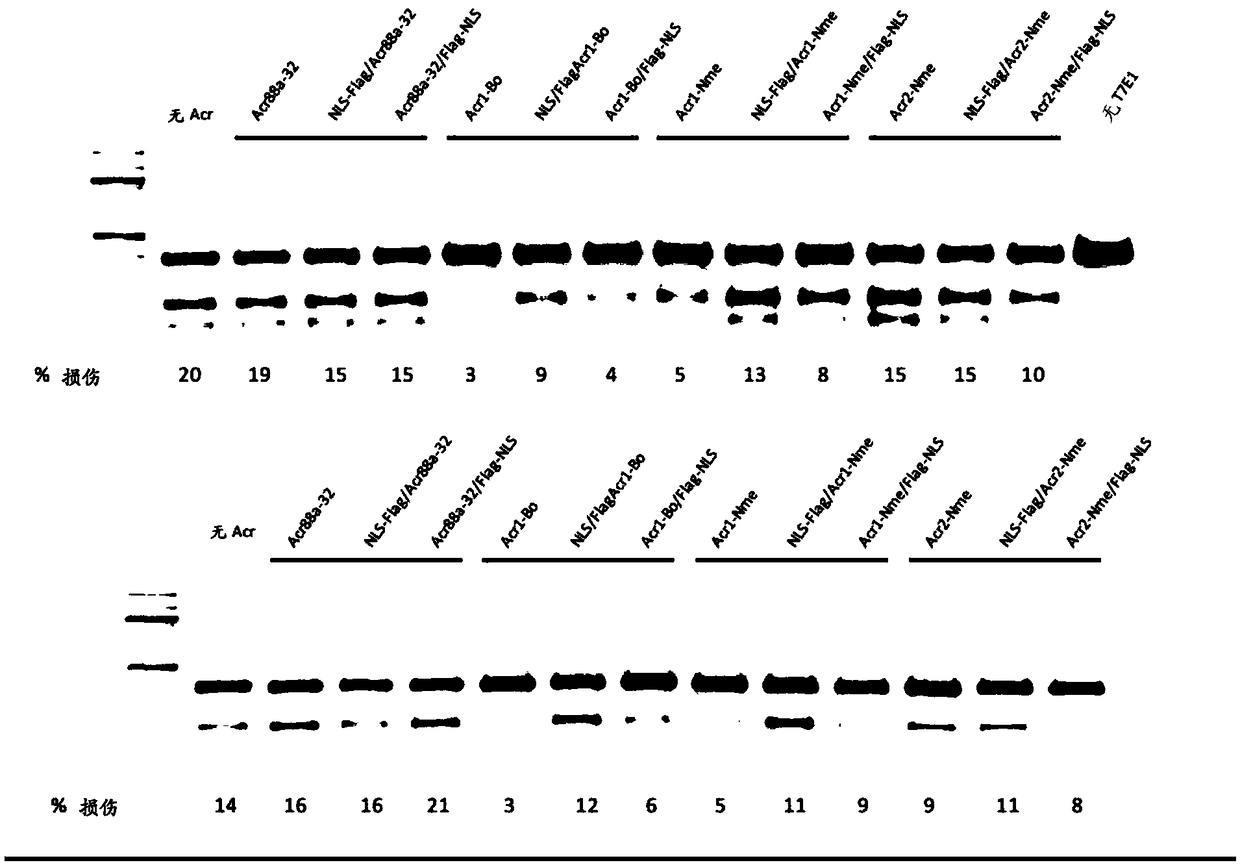
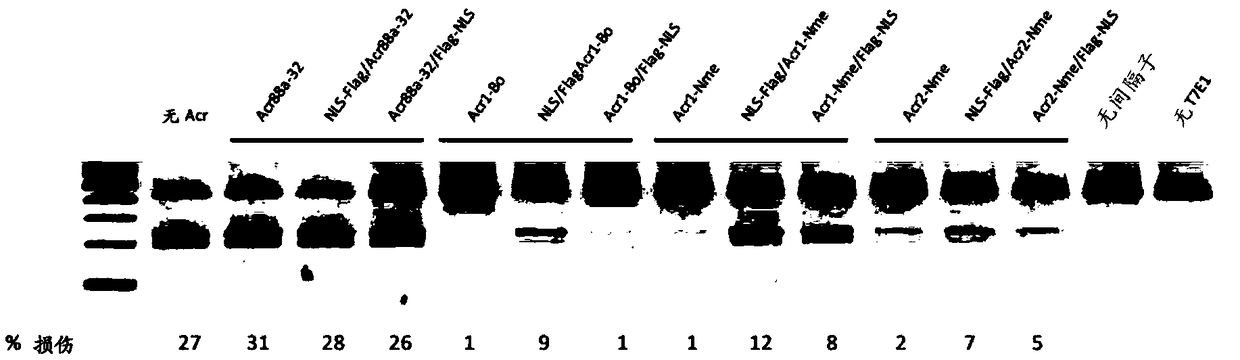
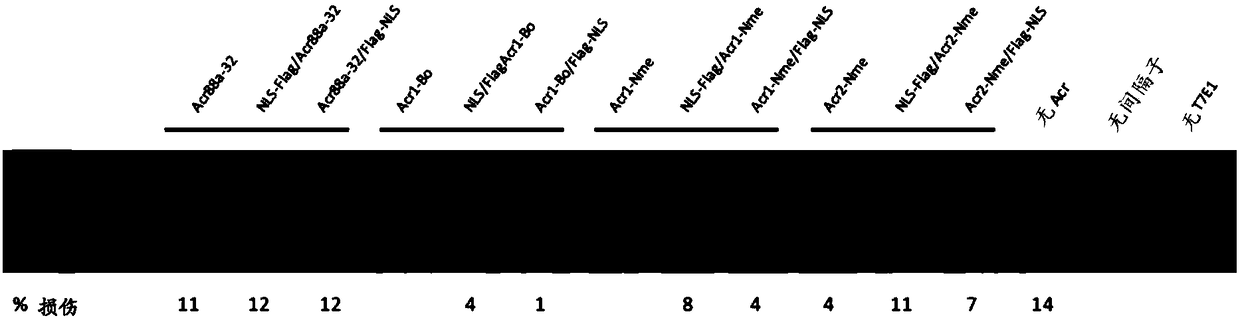
[0350] Many other acr gene candidates can be identified by their genetic association with aca genes or putative aca genes. The Acr open reading frame can be cloned directly from the species of origin, or alternatively, the gene sequence can be synthesized by a company. There are several approaches to screen for Acr activity against type II systems. 1) a test for the inhibition of NmeCas9 activity in its native context, namely in Neisseria meningitidis, by using the assay previously described to measure the transformation efficiency of DNA targeted by CRISPR-Cas9; 2) for Test of inhibition of NmeCas9 activity in a heterologous E. coli system (wherein NmeCas9 is programmed to target coliphage (eg, lambda phage)); 3) by using the T7E1 assay described herein, with respect to Test for inhibition of NmeCas9 activity in cell culture.
Embodiment III
[0352] Acr administration for controlled gene drive spread
[0353]Cas9 is currently being considered for use in gene drives, engineered genetic elements that can spread rapidly throughout natural populations. If that natural population is an undesired population, such as insects that transmit pathogens such as malaria parasites or dengue virus, the gene drive may include elements that induce death or sterility for any insects that acquire the gene drive. While the potential of this method for controlling insect-borne diseases is widely recognized, there are many concerns about its safety and potential for undesired adverse consequences (predictable or unpredictable) following release into the natural environment. worry. Part of that concern arises from the possibility that, once released, the spread of a gene drive may be difficult or impossible to stop. It is conceivable to imagine a gene drive that uses a type II-C Cas9 enzyme, such as NmeCas9, to support its propagatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com