Binary mercaptan oligomer as well as preparation method and application thereof
A technology of binary mercaptan and oligomers, applied in the field of binary mercaptan oligomers and its preparation, can solve problems such as bad smell of resin, achieve small volume shrinkage, weaken mercaptan volatility, and fast polymerization rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
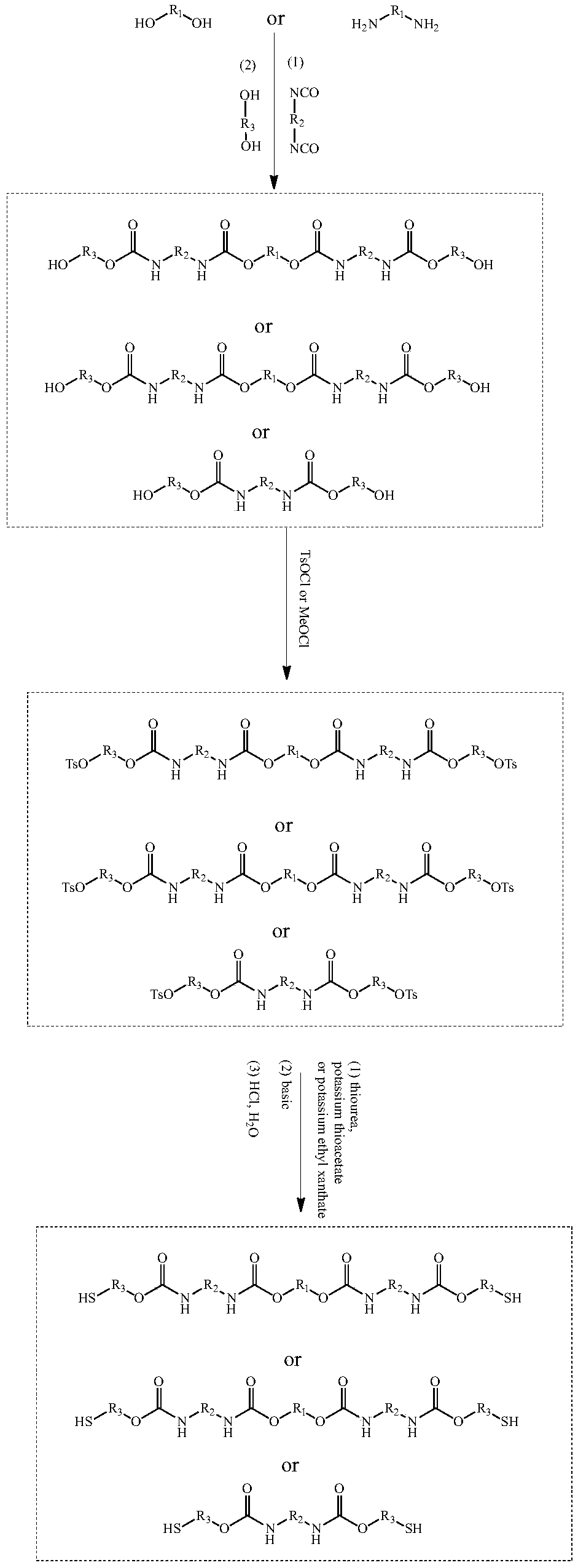
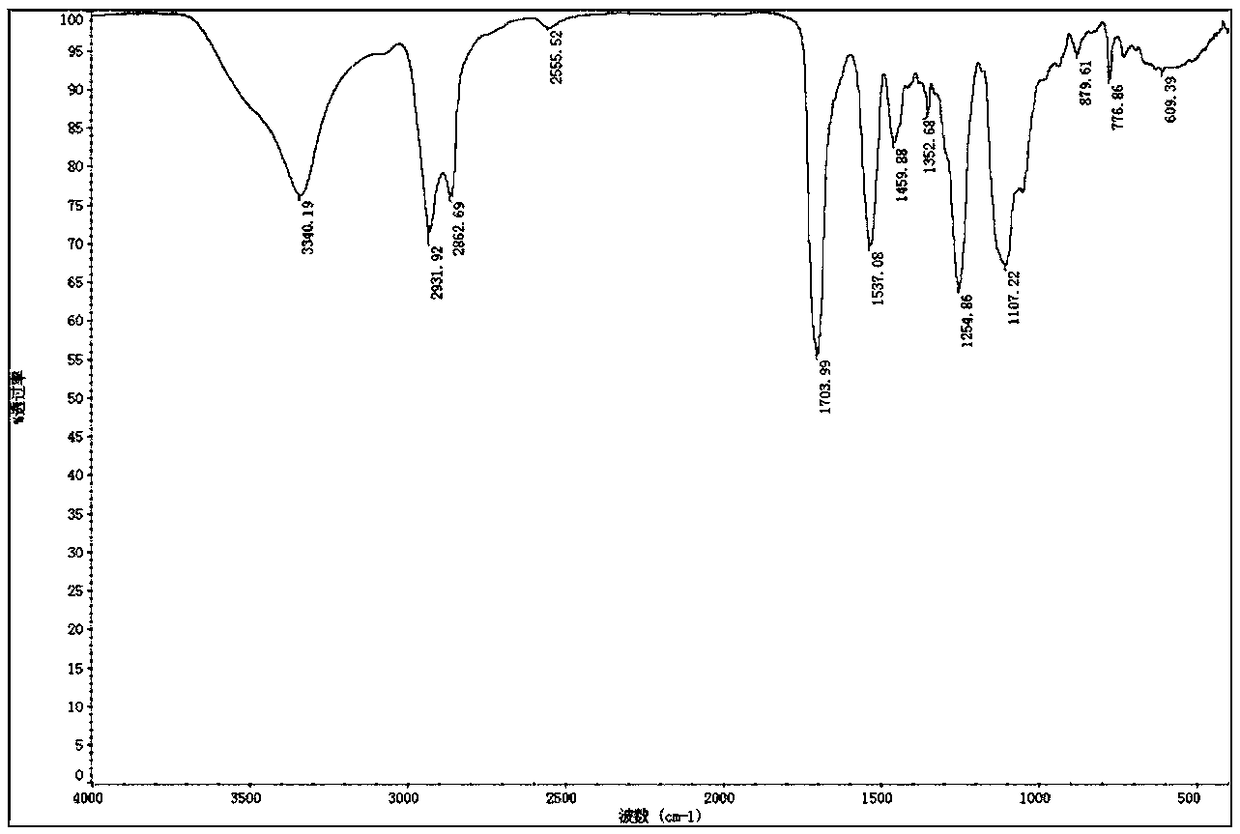
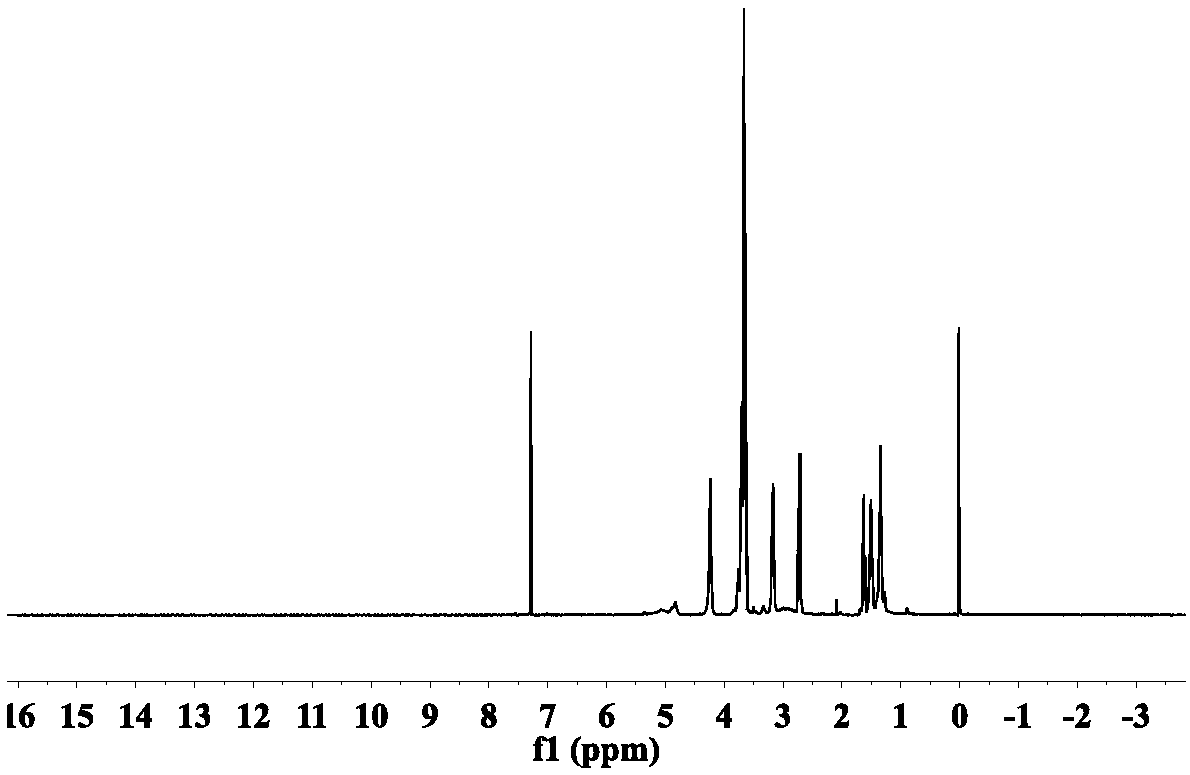
[0100] This embodiment provides a kind of dibasic thiol oligomer: polyurethane dibasic thiol monomer T1 and a kind of photosensitive resin system S1, polyurethane dibasic thiol monomer T10 and photosensitive resin system S1 are prepared by following steps (such as figure 1 ).
[0101] (1) Preparation of Polyurethane Dibasic Mercaptan Monomer T1
[0102] S1: Polyurethane diol PU 1 Preparation of -OH
[0103] First 200g of hexamethylene diisocyanate (HDI) and 1.2g of triethylamine were dissolved in tetrahydrofuran, and then 230g of polyethylene glycol (number average molecular weight 200, hydroxyl value 580mg / g) was added dropwise to In the reaction solution, continue to react at 0°C for 12 hours, follow the reaction with infrared until the isocyanate group (-NCO) is at 2230cm -1 The absorption peaks at the left and right sides disappear, indicating that the reaction is complete. Underpressure distillation removes solvent and removes and obtains polyurethane binary oligomer ...
Embodiment 2
[0114] This embodiment provides a dibasic thiol oligomer: a polyurethane dibasic thiol monomer T2 and a photosensitive resin system S2. The polyurethane dibasic thiol monomer T2 and the photosensitive resin system S2 are prepared through the following steps.
[0115] (1) Preparation of Polyurethane Dibasic Mercaptan Monomer T2
[0116] S1: Polyurethane PU 2 Preparation of -OH
[0117] First the dodecamethylene diisocyanate (HDI) of 200g and the triethylamine of 0.84g are dissolved in tetrahydrofuran, the ethylene glycol of 51.7g is slowly added dropwise, then the polyethylene glycol of 1557g (number average molecular weight 2000, 60mg / g), added dropwise to the reaction solution, continued to react at 0°C for 12 hours, followed the reaction with infrared until the isocyanate group (-NCO) was at 2230cm -1 The absorption peaks at the left and right sides disappear, indicating that the reaction is complete. Underpressure distillation removes solvent and removes and obtains poly...
Embodiment 3
[0126] This embodiment provides a dibasic thiol oligomer: a polyurethane dibasic thiol monomer T3 and a photosensitive resin system S3. The polyurethane dibasic thiol monomer T3 and the photosensitive resin system S3 are prepared through the following steps.
[0127] (1) Preparation of Polyurethane Dibasic Mercaptan Monomer T3
[0128] S1: Polyurethane PU 3 Preparation of -OH
[0129] Dissolve 200g of 1,4-diisocyanatocyclohexane and 1.2g of triethylamine in tetrahydrofuran, slowly add 142g of hexanediol dropwise, and react at 50°C for 12 hours, then add 482g of polyethylene glycol Alcohol (number-average molecular weight 400, hydroxyl value 280mg / g), was added dropwise into the reaction solution, continued to react at 50°C for 12 hours, followed the reaction with infrared until the isocyanate group (-NCO) was at 2230cm -1 The absorption peaks at the left and right sides disappear, indicating that the reaction is complete. Underpressure distillation removes solvent and remov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com