Photosensitive resin composition for stereolithography
A technology of photosensitive resin and composition, applied in the field of polymer materials, can solve the problems of unsolved odor of thiol monomer, hindering the application of thiol-olefin photosensitive resin, unpleasant smell of small molecule thiol monomer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
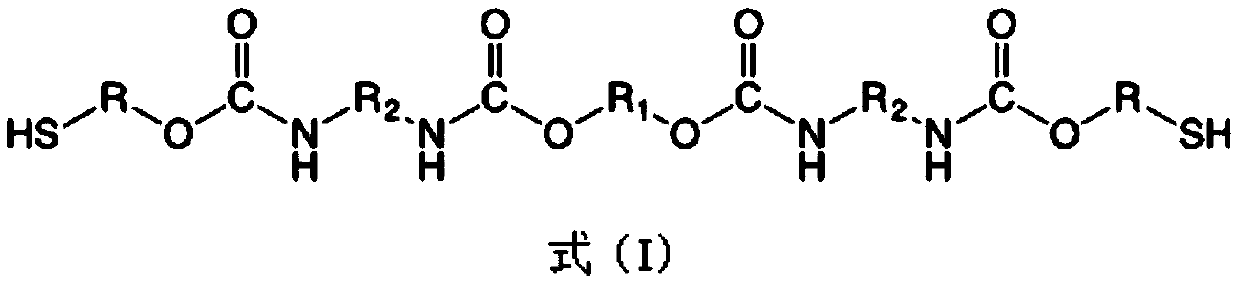
[0072] This embodiment provides a polyurethane dihydric thiol prepolymer (odorless polyurethane thiol monomer T1) and a photosensitive resin (photosensitive resin system S1) prepared therefrom.
[0073] The composition (mass fraction) of the photosensitive resin system S1 is as follows: polyamic acid dibasic thiol monomer T1 60%, tetrakis (ethylene glycol) diacrylate 38%, hydroquinone 1%, 2-hydroxy-2- Methyl-p-hydroxyethyl ether phenylacetone 1%.
[0074] Odorless polyurethane thiol monomer T1 and photosensitive resin system S1 can be prepared by the following method:
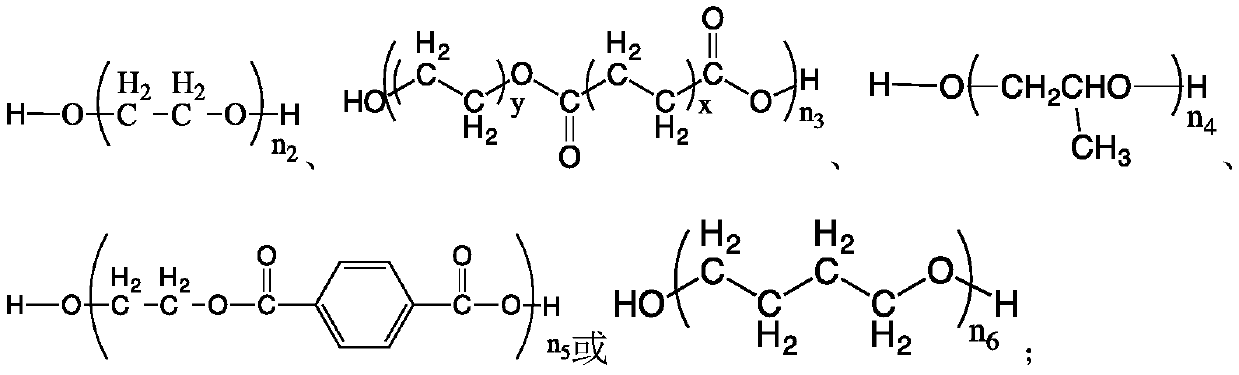
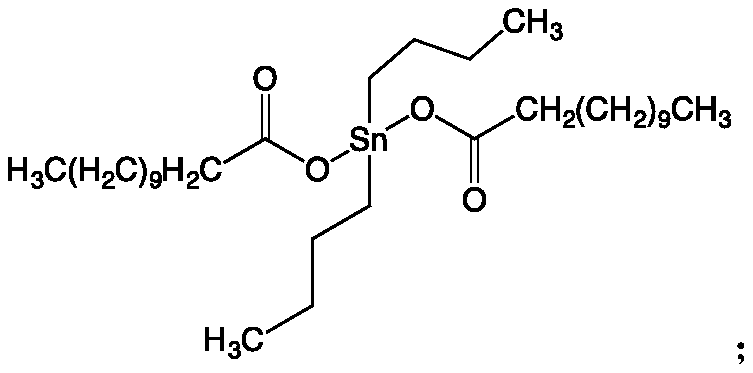
[0075] (1) Synthesis of odorless polyurethane thiol monomer T1: In a three-necked round-bottomed flask of 1000mL, under nitrogen protection, 181g of 6-bromohexanol, 147g of isophorone diisocyanate (IPDI) and 0.97g of dibutyltin dilaurate, dissolved in 200mL of acetone, reacted at 50°C for 5 hours, then added 21g of polyethylene glycol (molecular weight: 200), raised the temperature to 75°C and continued to rea...
Embodiment 2
[0078] This embodiment provides a polyurethane dihydric thiol prepolymer (odorless polyurethane thiol monomer T2) and a photosensitive resin (photosensitive resin system S2) prepared therefrom.
[0079] The composition (mass fraction) of the photosensitive resin system S2 is as follows: polyamic acid dibasic thiol monomer T2 50%, tetra(ethylene glycol) diacrylate 30%, tripropoxylated glycerol triacrylate 10% , Hydroquinone 5%, 2-hydroxy-2-methyl-p-hydroxyethyl ether phenylacetone 5%.
[0080] Odorless polyurethane thiol monomer T2 and photosensitive resin system S2 can be prepared by the following method:
[0081] (1) Synthesis of odorless polyurethane thiol monomer T2: In a 1000mL three-necked round-bottomed flask, under nitrogen protection, 150g of 2-bromoethanol, 300g of diphenylmethane diisocyanate (MDI) and 0.97 2 g of dibutyltin dilaurate, dissolved in 200 mL of acetone, reacted at 50 ° C for 5 hours, then added 290 g of polyethylene glycol (molecular weight 2000), rais...
Embodiment 3
[0084] This embodiment provides a polyurethane dihydric thiol prepolymer (odorless polyurethane thiol monomer T3) and a photosensitive resin (photosensitive resin system S3) prepared therefrom.
[0085] Odorless polyurethane thiol monomer T3 and photosensitive resin system S3 can be prepared by the following method:
[0086] The composition (mass fraction) of the photosensitive resin system S3 is as follows: polyamic acid dibasic thiol monomer T3 60%, tetra(ethylene glycol) diacrylate 30%, tripropoxylated glycerol triacrylate 6% , Hydroquinone 2%, 2-hydroxy-2-methyl-p-hydroxyethyl ether phenylacetone 2%.
[0087] (1) Synthesis of odorless polyurethane thiol monomer T3: in a three-necked round-bottomed flask of 1000mL, under nitrogen protection, 120g of 6-bromohexanol, 147g of dicyclohexylmethane diisocyanate (HMDI) and 0.2 g of dibutyltin dilaurate was dissolved in 200 mL of acetone, and reacted at 50° C. for 7 hours, then 620 g of polyethylene glycol oxalate (molecular weigh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com