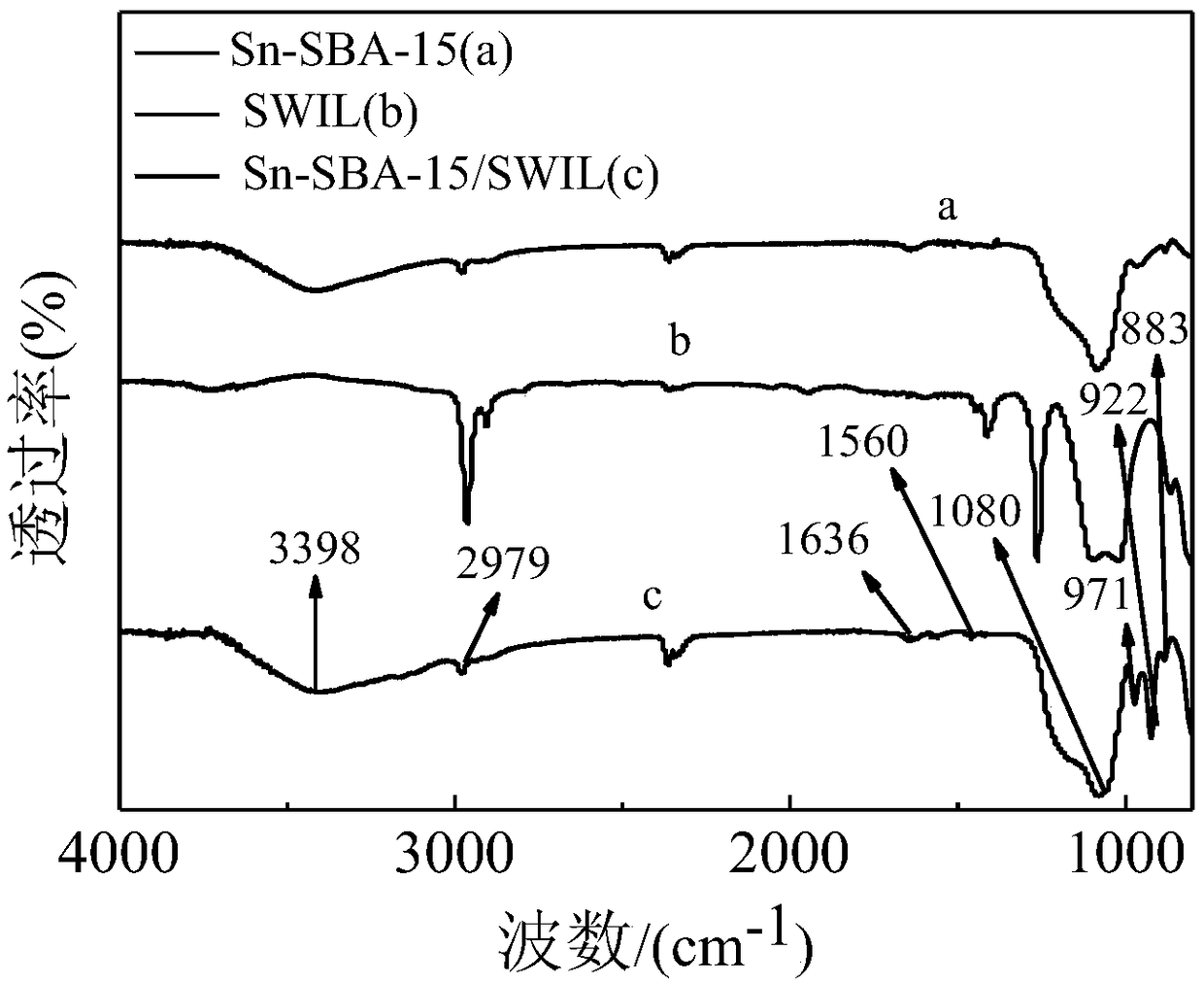
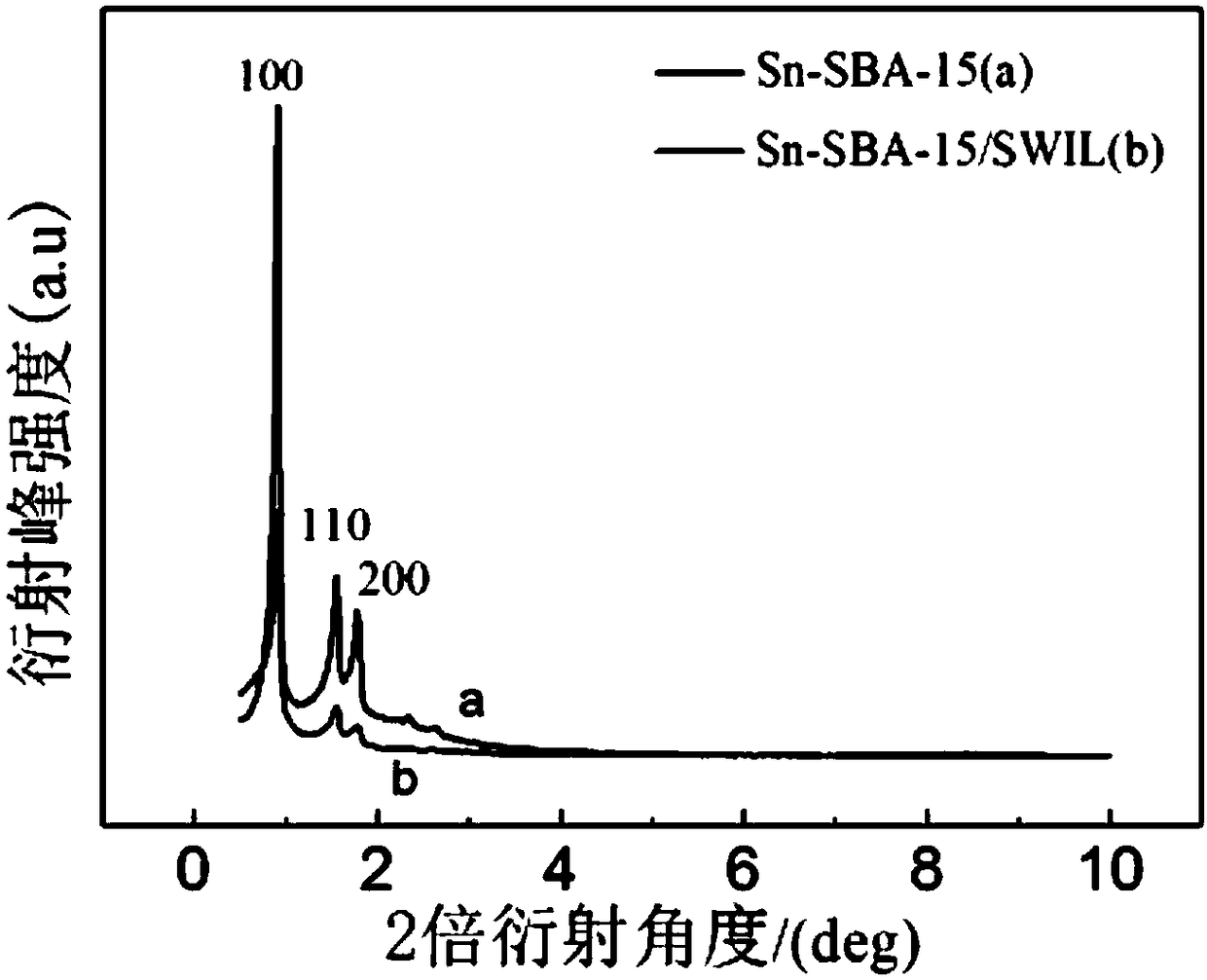
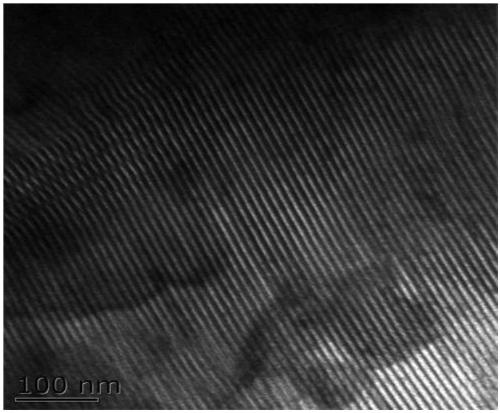
Preparation method of heteropolyacid ionic liquid-loaded Sn-SBA-15 catalyst and application of catalyst
A technology of sn-sba-15 and ionic liquid, which is applied in the field of preparation of Sn-SBA-15 catalyst, can solve problems such as catalytic activity to be optimized, and achieve the effects of improving utilization rate, overcoming recovery difficulties, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Firstly, the heteropolyacid ionic liquid and Sn-SBA-15 were prepared respectively, and the ionic liquid was combined with the carrier by impregnation method to obtain the supported heteropolyacid ionic liquid catalyst. The specific method is as follows:
[0037] 1. Preparation of Sn-SBA-15
[0038] Weigh 1.5g of P123 and dissolve in 70ml of deionized water, add 1.18g of NaCl solid, and stir mechanically at 35-40°C until clear. Slowly drop 4.2g of TEOS at 35°C for 4h in advance. Add 0.35g of SnCl 4 ·5H 2 O The white solid (Si / Sn=20) continued to be mechanically stirred for 24h, forming a white cloudy liquid. The reaction mixture was transferred to polytetrafluoroethylene for hydrothermal crystallization for 24h. Suction filtration, washing and drying. Put it in a tube furnace, raise the temperature to 500°C at a heating rate of 1°C / min, and calcine in air for 12 hours to remove the template agent, and obtain the Sn-SBA-15 mesoporous material.
[0039] Step 2. Prep...
Embodiment 2
[0050] According to the reaction conditions of Step 1 and Step 2 in Example 1, keep the other conditions of Step 3 unchanged, and change the reflux reaction temperature to 60°C.
[0051] Gained catalyst is applied in the esterification reaction of acetic acid and benzyl alcohol, reaction temperature is 100 ℃, acetic acid 6.0g, benzyl alcohol 8.1g, catalyzer 0.4g, the relation of acetic acid conversion rate and time is as follows Figure 7 As shown, the reaction can reach equilibrium in 5h, and the maximum conversion rate of acetic acid can reach 85.68%. It can be seen that when the reflux reaction temperature is 60°C, the catalytic activity of the obtained catalyst is slightly lower than that of the catalyst prepared at 50°C. Considering the energy loss problem, we take the reflux reaction temperature as 50°C. Example 3
Embodiment 3
[0052] After determining the ideal temperature for the composite of the carrier and the ionic liquid, continue to explore the influence of the doping amount of the ionic liquid in the carrier Sn-SBA-15 on the catalyst activity. According to the method of steps 1 and 2 in Example 1, keep other conditions unchanged. change, respectively by m SWIL / m Sn-SBA-15 =0.8, 1, 1.2, 1.4 mass ratio to treat the catalyst, and the obtained catalysts are respectively named as C1, C2, C3, and C4. And use these catalysts to catalyze the esterification reaction of acetic acid and benzyl alcohol, the conditions are the same as in Example 1, and the conversion rate of acetic acid is as follows: Figure 8 As shown, prove that the equivalent m SWIL / m Sn-SBA-15 =1.2, the conversion of acetic acid reaches the maximum. Continuing to increase the doping amount of ionic liquid, the conversion rate decreased slightly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com