Method for synthesizing allyl phenoxyacetate
A synthesis method and technology of pineapple ether, applied in the field of organic synthesis, can solve the problems of equipment corrosion, large amount of waste water, and difficult treatment, and achieve the effects of no serious corrosion, improved selectivity, and improved conversion rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
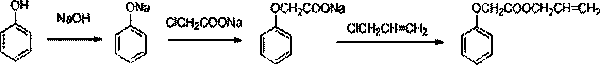
[0026] Drop into phenol 94g (1mol), caustic soda 48g (1.2mol), water 195g in the flask, heat up, control 80 ℃~90 ℃ and stir reaction 2 hours, normal pressure sloughs off 100g water, cools down to 50 ℃, drops into toluene 500g, Raise the temperature, and use toluene to carry out the water to obtain a suspension of sodium phenate in toluene.
[0027] 116.5 g (1 mol) of sodium chloroacetate was dropped into the toluene suspension of the above-mentioned sodium phenate, and the temperature was raised to reflux for 4 hours to obtain a toluene suspension of sodium phenoxyacetate.
[0028] Add 114.75 g (1.5 mol) of allyl chloride and 4.7 g of benzyltriethylammonium chloride into the toluene suspension of sodium phenoxyacetate, and heat up and reflux for 6-10 hours until the content of allyl chloride does not change when analyzed by GC After finishing the reaction, the temperature was lowered, and the catalyst and the sodium chloride produced by the reaction were filtered off to obtain...
Embodiment 2
[0031] Put 94g (1mol) of phenol, 40g (1mol) of caustic soda, and 120g of water into the flask, heat up, control 80°C to 90°C to stir and react for 1 hour, remove 60g of water under normal pressure, cool to 50°C, add 470g of toluene, and heat up , Use toluene to bring the water to the end to obtain a suspension of sodium phenate in toluene.
[0032] 139.8 g (1.2 mol) of sodium chloroacetate was dropped into the toluene suspension of the above-mentioned sodium phenate, and the temperature was raised to reflux for 3 hours to obtain a toluene suspension of sodium phenoxyacetate.
[0033] Add 133.88 g (1.75 mol) of allyl chloride and 2.82 g of benzyltriethylammonium bromide into the toluene suspension of sodium phenoxyacetate, and heat up and reflux for 6-10 hours until the content of allyl chloride does not change when analyzed by GC After finishing the reaction, the temperature was lowered, and the catalyst and the sodium chloride produced by the reaction were filtered off to obt...
Embodiment 3
[0036] Drop into phenol 94g (1mol), caustic soda 60g (1.5mol), water 300g in the flask, heat up, control 80 ℃~90 ℃ stirring reaction 3 hours, normal pressure sloughs off 180g water, cools down to 50 ℃, drops into toluene 517g, Raise the temperature, and use toluene to carry out the water to obtain a suspension of sodium phenate in toluene.
[0037] 174.8 g (1.5 mol) of sodium chloroacetate was dropped into the toluene suspension of the above-mentioned sodium phenate, and the temperature was raised to reflux for 5 hours to obtain a toluene suspension of sodium phenoxyacetate.
[0038] Add 153 g (2 mol) of allyl chloride and 7.52 g of benzyltriethylammonium chloride into the toluene suspension of sodium phenoxyacetate, heat up and reflux for 6-10 hours, and end the reaction when the content of allyl chloride does not change after GC analysis , lower the temperature, filter off the catalyst and the sodium chloride produced by the reaction, and obtain the crude product containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com