Lithium oxygen battery electrolyte using molybdenum pentachloride as redox medium and preparation and application thereof
A lithium-oxygen battery and molybdenum pentachloride technology, applied in the field of electrochemical catalysis, can solve the problems of unstable and easy decomposition of organic compounds, unsatisfactory catalytic performance of catalysts, and battery performance decline, so as to stabilize the battery system and reduce charging Voltage, the effect of improving the cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Dry molybdenum pentachloride in a vacuum desiccator at 120°C for 24 hours, then dissolve molybdenum pentachloride in tetraethylene glycol with a lithium bistrifluoromethylsulfonimide concentration of 1 mol / L in a glove box In the electrolytic solution of dimethyl ether, the electrolytic solution using molybdenum pentachloride as redox medium can be obtained in the present invention, wherein the concentration of molybdenum pentachloride is 0.05 mol / L.
Embodiment 2
[0027] Molybdenum pentachloride was dried in a vacuum desiccator at 120 °C for 24 hours, and then the electrolysis of molybdenum pentachloride dissolved in lithium trifluoromethanesulfonate with a concentration of 1 mol / L ethylene glycol dimethyl ether in a glove box In the liquid, the electrolytic solution using molybdenum pentachloride as redox medium can be obtained in the present invention, wherein the concentration of molybdenum pentachloride is 0.05 mol / L.
Embodiment 3
[0029] Molybdenum pentachloride was dried in a vacuum desiccator at 120°C for 24 hours, and then molybdenum pentachloride was dissolved in an electrolyte solution of tetraethylene glycol dimethyl ether with a lithium trifluoroacetate concentration of 0.5 mol / L in a glove box In the present invention, the electrolytic solution using molybdenum pentachloride as a redox medium can be obtained, wherein the concentration of molybdenum pentachloride is 0.05 mol / L.
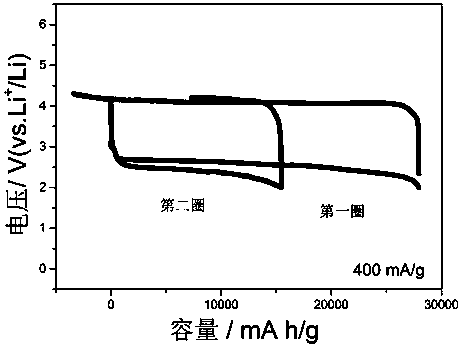
[0030] figure 1 The charge-discharge curves of lithium-oxygen batteries added molybdenum pentachloride at a current density of 400 mA / g. It can be seen from the figure that the lithium-oxygen battery containing the redox medium of molybdenum pentachloride can discharge up to 27951 mA h / g in the first week, the charging voltage can be reduced to 4.0 V, and the capacity can still reach 15731 mA h in the second cycle / g, and the Faradaic efficiency reaches over 80%, showing good reversibility.
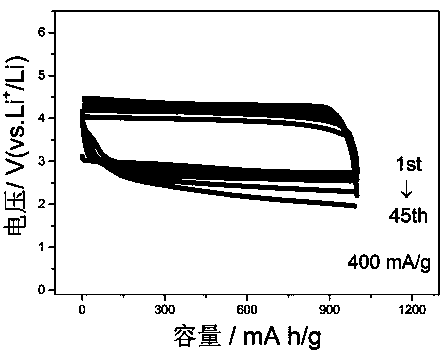
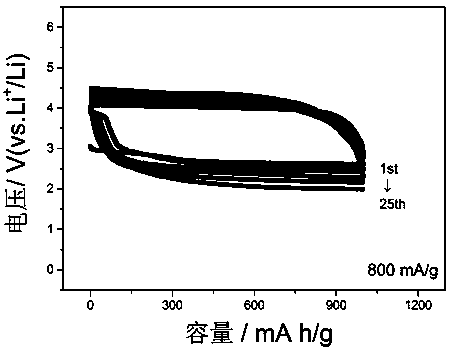
[0031] figure 2 It is th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com