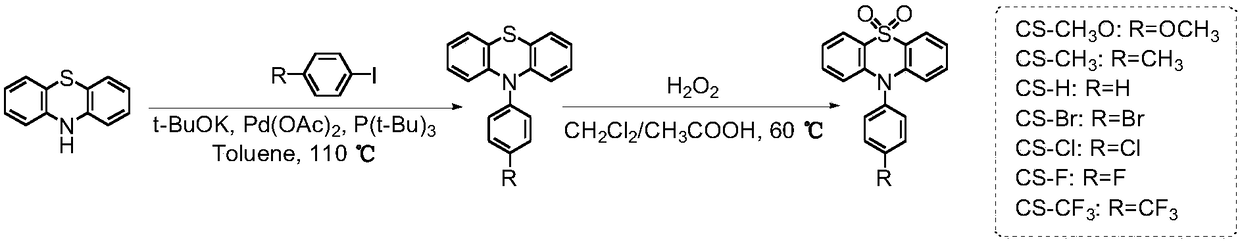
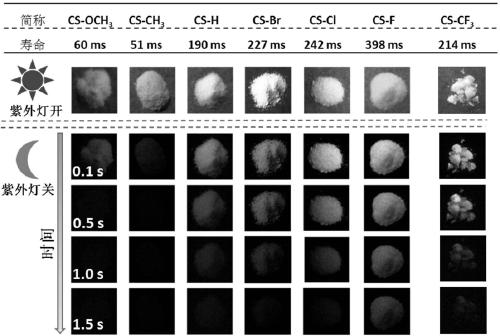
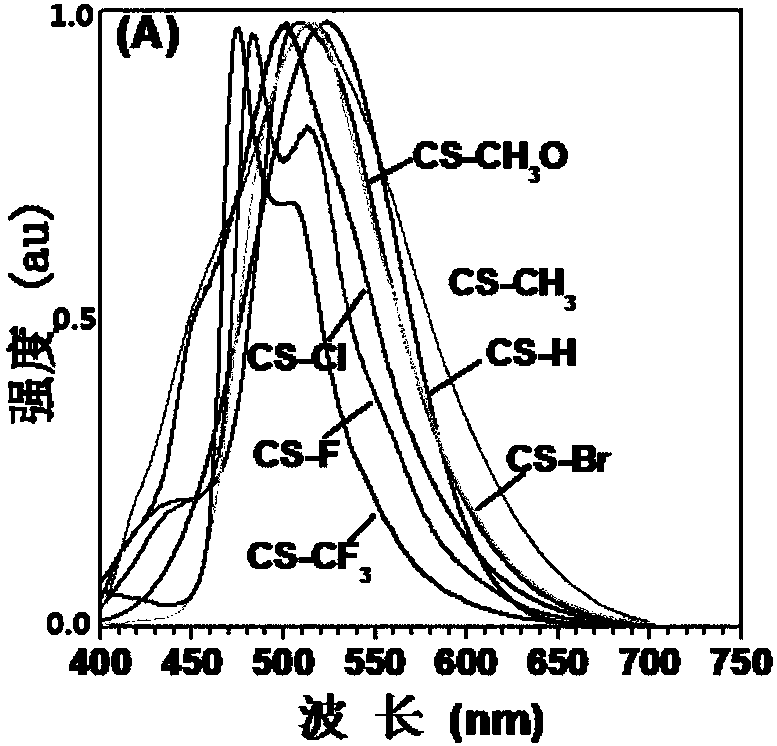
Room temperature phosphorescence molecule based on phenothiazine derivative, preparation method and application thereof
A phenothiazine derivative and room temperature phosphorescence technology, which is applied in the application of anti-counterfeiting materials, room temperature phosphorescence molecules and their preparation fields, can solve the problems of scarcity and few types of pure organic room temperature phosphorescence materials, and achieves high yield and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Compound CS-CH 3 Synthesis of O
[0030] Under nitrogen protection, in a reaction flask, phenothiazine (1.99g, 10mmol), 4-methoxyiodobenzene (2.34g, 12mol), potassium tert-butoxide (1.68g, 15mmol) and palladium acetate (0.11 g, 0.5mmol), tri-tert-butylphosphine (0.5mL, 0.25mmol) were dissolved in a sufficient amount of toluene solution, stirred and refluxed at 110-120°C for 12h, after the reaction was completed, the reaction solution was cooled to room temperature, and then used Extract with chloroform, collect the organic phase, and then use anhydrous Na 2 SO 4 Dried and spin-dried to obtain a crude product. Using petroleum ether as eluent, the crude product was separated and purified by silica gel column chromatography, and then the obtained product was dissolved in a mixed solution of dichloromethane (90 mL), acetic acid (45 mL) and hydrogen peroxide (2 mL), and dissolved at 70 ℃ for 12 h, the reaction solution was extracted with chloroform, the organi...
Embodiment 2
[0033] Example 2: Compound CS-CH 3 Synthesis
[0034] Under nitrogen protection, in a reaction flask, phenothiazine (1.99g, 10mmol), 4-methyliodobenzene (2.62g, 12mol), potassium tert-butoxide (1.68g, 15mmol) and palladium acetate (0.11g , 0.5mmol), tri-tert-butylphosphine (0.5mL, 0.25mmol) were dissolved in a sufficient amount of toluene solution, stirred and refluxed at 110-120°C for 15h, after the reaction was completed, the reaction solution was cooled to room temperature, and then used three Extract with methyl chloride, collect the organic phase, and then use anhydrous Na 2 SO 4 Dried and spin-dried to obtain a crude product. Using petroleum ether as eluent, the crude product was separated and purified by silica gel column chromatography, and then the obtained product was dissolved in a mixed solution of dichloromethane (90 mL), acetic acid (45 mL) and hydrogen peroxide (2 mL), and dissolved at 70 ℃ for 16 h, the reaction solution was extracted with chloroform, the o...
Embodiment 3
[0037] Embodiment 3: the synthesis of compound CS-H
[0038] Under nitrogen protection, in a reaction flask, phenothiazine (1.99g, 10mmol), iodobenzene (2.45g, 12mol), potassium tert-butoxide (1.68g, 15mmol) and palladium acetate (0.11g, 0.5mmol) , Tri-tert-butylphosphine (0.5mL, 0.25mmol) was dissolved in a sufficient amount of toluene solution, stirred and refluxed at 110-120°C for 20h, after the reaction was completed, the reaction solution was cooled to room temperature, and then extracted with chloroform, The organic phase was collected and washed with anhydrous Na 2 SO 4 Dried and spin-dried to obtain a crude product. Using petroleum ether as eluent, the crude product was separated and purified by silica gel column chromatography, and then the obtained product was dissolved in a mixed solution of dichloromethane (90 mL), acetic acid (45 mL) and hydrogen peroxide (2 mL), and dissolved at 70 ℃ for 18 h, the reaction solution was extracted with chloroform, the organic ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com