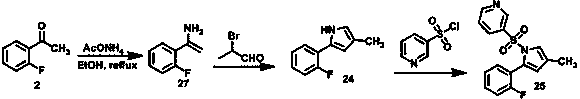
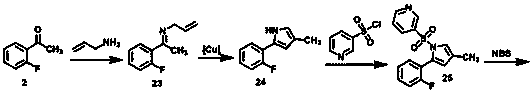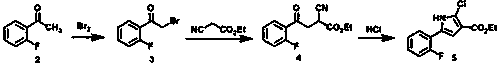Preparation method of vonoprazan
A synthesis method and fluorophenyl technology, applied in the field of medicine, can solve the problems of unfriendly environment, long synthesis route, unstable intermediate and the like, and achieve the effects of convenient industrial production, convenient quality control and few synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] (1) Preparation of ethyl 4-(2-fluorophenyl)-2-formyl-4-oxobutanoate.
[0093] Add ethyl 3-oxopropionate (12.8 g, 110 mmol) and tetrahydrofuran (80 mL) to a 250 mL reaction flask, add sodium ethoxide (7.5 g, 110 mmol) in batches, stir at room temperature for 0.5 hours, add dropwise 2- Bromo-2'-fluoroacetophenone (21.7g, 100 mmol) in tetrahydrofuran (20 mL) was reacted at room temperature for 4 hours. Concentrate under reduced pressure, add water (100 mL), stir at room temperature for 0.5 hours, and filter to obtain ethyl 4-(2-fluorophenyl)-2-formyl-4-oxobutanoate (yield: 97.9%, HPLC purity : 98.88%).
[0094] (2) Preparation of ethyl 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-pyrrole-3-carboxylate
[0095] Add ethyl 4-(2-fluorophenyl)-2-formyl-4-oxobutanoate (20.2 g, 80 mmol), pyridine-3-sulfonamide (15.2 g, 96 mmol) into a 250 mL reaction flask ) and glacial acetic acid (100 mL), reacted at 100°C for 12 hours, and concentrated under reduced pressure. Add water (100 ...
Embodiment 2
[0102] (1) Preparation of ethyl 4-(2-fluorophenyl)-2-formyl-4-oxobutanoate.
[0103] Add ethyl 3-oxopropionate (12.8 g, 110 mmol) and tetrahydrofuran (80 mL) to a 250 mL reaction flask, add sodium ethoxide (6.8 g, 100 mmol) in batches, stir at room temperature for 0.5 hours, add dropwise 2- Bromo-2'-fluoroacetophenone (21.7g, 100 mmol) in tetrahydrofuran (20 mL) was reacted at room temperature for 4 hours. Concentrate under reduced pressure, add water (100 mL), stir at room temperature for 0.5 hours, and filter to obtain ethyl 4-(2-fluorophenyl)-2-formyl-4-oxobutanoate (yield: 93.6%, HPLC purity : 98.29%).
[0104] (2) Preparation of ethyl 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-pyrrole-3-carboxylate
[0105]Add ethyl 4-(2-fluorophenyl)-2-formyl-4-oxobutanoate (20.2 g, 80 mmol), pyridine-3-sulfonamide (15.2 g, 96 mmol) into a 250 mL reaction flask ) and glacial acetic acid (100 mL), reacted at 80°C for 12 hours, and concentrated under reduced pressure. Add water (100 mL...
Embodiment 3
[0112] (1) Preparation of ethyl 4-(2-fluorophenyl)-2-formyl-4-oxobutanoate.
[0113] Add ethyl 3-oxopropionate (12.8 g, 110 mmol), tetrahydrofuran (80 mL) and triethylamine (7.5 g, 110 mmol) into a 250 mL reaction flask, stir at room temperature for 0.5 hours, then add 2-bromo - 2'-fluoroacetophenone (21.7 g, 100 mmol) in tetrahydrofuran (20 mL), react at room temperature for 4 hours. Concentrate under reduced pressure, add water (100 mL), stir at room temperature for 0.5 hours, and filter to obtain ethyl 4-(2-fluorophenyl)-2-formyl-4-oxobutanoate (yield: 93.2%, HPLC purity : 98.49%).
[0114] (2) Preparation of ethyl 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-pyrrole-3-carboxylate
[0115] Add ethyl 4-(2-fluorophenyl)-2-formyl-4-oxobutanoate (20.2 g, 80 mmol), pyridine-3-sulfonamide (15.2 g, 96 mmol) into a 250 mL reaction flask ) and glacial acetic acid (100 mL) at 100°C for 6 hours. Concentrate under reduced pressure, add water (100 mL), adjust its pH to 9 with saturate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com