An anti-respiratory syncytial virus drug for patients with cardiovascular disease
A syncytial virus and anti-respiratory technology, applied in the field of medicine, can solve problems such as the inability to use ribavirin and myocardial damage, and achieve the effects of easy production and promotion, enhanced inhibitory effect, and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Derivative Preparation
[0028] In a single-necked bottle, mix substituted phenylacetic acid (0.01mol), substituted benzaldehyde (0.01mol), acetic anhydride 6.0mL (freshly distilled) and triethylamine 0.7mL (freshly distilled), and heat Reflux reaction in the bag for 4 to 6 hours, lower the temperature to 100°C, add 1 to 2 mL of distilled water to decompose, and then stir for 5 minutes, remove the reactant from the electric heating bag, cool to room temperature, put it into 60 mL of water, leave it at room temperature to precipitate solids, and filter The product was obtained as a solid.
[0029] For the solid product in the previous step, if adding NaOH solution, adjust the pH at about 12, stir and react at 90°C for 2 hours, cool to room temperature, adjust the pH at 6-7 with dilute HCl solution, precipitate off-white solid, and filter to obtain the product , E-configuration derivatives A1, A4, A5 and A6 with phenolic hydroxyl groups can be prepared. The pr...
Embodiment 2
[0042] Embodiment 2 Biological test
[0043] 1. Screening for antiviral activity
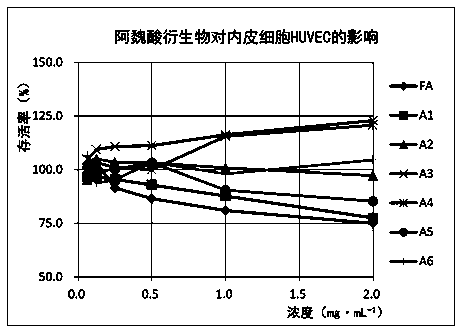
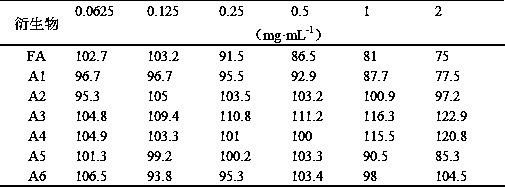
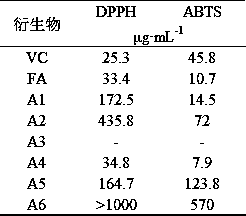
[0044] In vitro antiviral effect of ferulic acid derivatives, common respiratory syncytial virus (RSV), herpes simplex virus type 1 (HSV-1), enterovirus type 71 (EV71) and other antiviral effect research, reference ( Yue Lulu et al., World Traditional Chinese Medicine, 2018, 13(01):199-206) method, observing the cell survival rate under a microscope. The content of this part is tested by Shandong Provincial Antiviral Research Center.
[0045] Ferulic acid (FA) and its derivatives (A1-A6) were prepared into the test solution. The experiment was divided into a cell control group, a virus control group and a drug group, and ribavirin was used as a reference. Three replicate wells were set up for each group. In the drug group, ferulic acid derivatives were diluted with cell maintenance solution, and serially diluted from 1:2, and added to the cultured monolayer human laryngeal carcinoma cells (H...
Embodiment 3
[0065] Embodiment 3 pharmaceutical composition preparation
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com