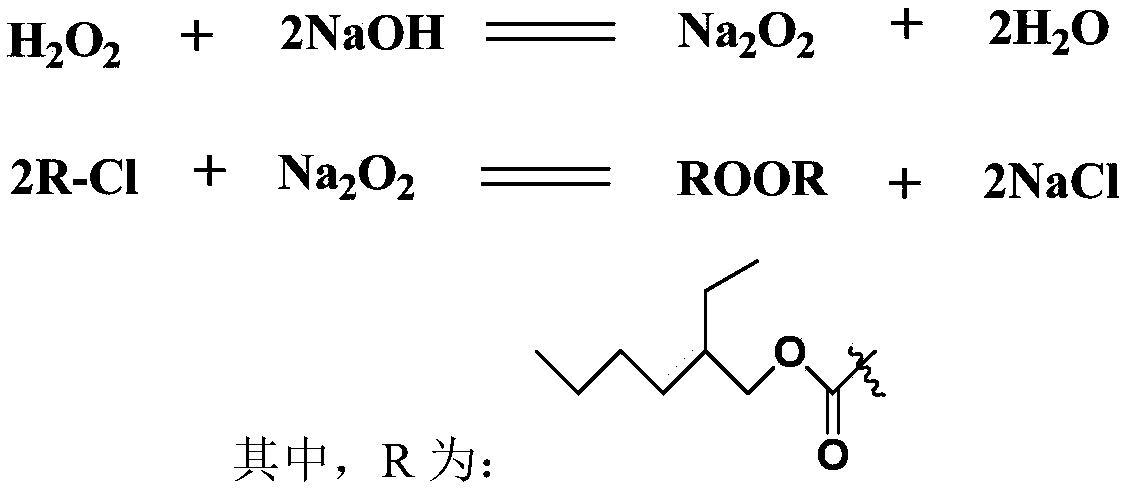Method for preparing di(2-ethylhexyl) peroxydicarbonate
A technology of dicarbonic acid peroxide and ethylhexyl, which is applied to the preparation of peroxy compounds, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of shortened operation difficulty, obvious amplification effect, and low decomposition temperature, and achieve reduction Effects of response time, high operational safety, and short response times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
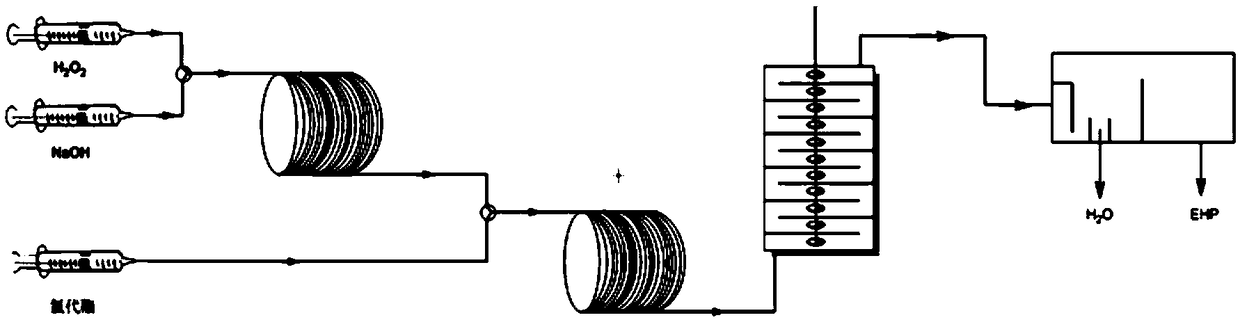
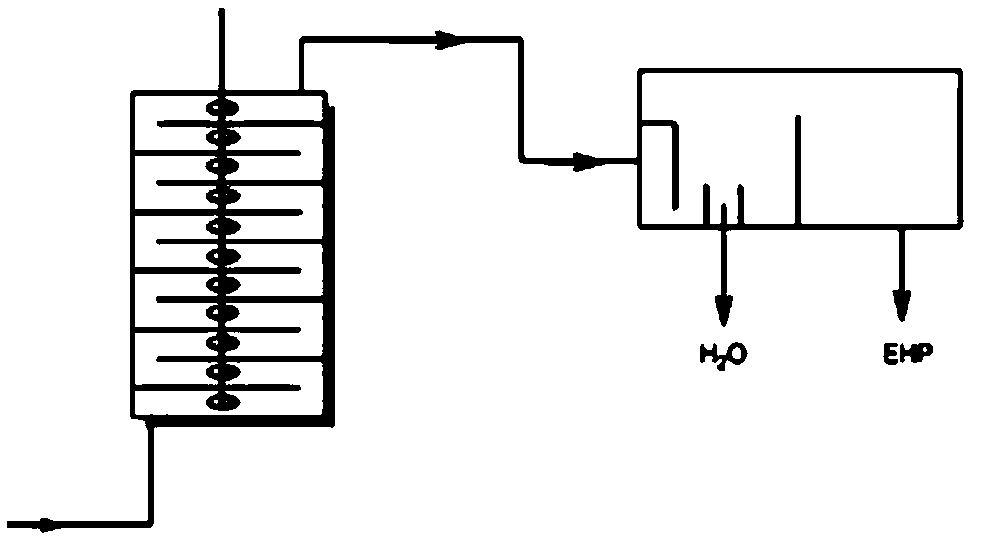
[0033] Prepare 20wt% hydrogen peroxide aqueous solution and 15wt% sodium hydroxide aqueous solution respectively, pump the 20wt% hydrogen peroxide aqueous solution and 15wt% sodium hydroxide aqueous solution into the first micro-mixer of the micro-reaction device, mix thoroughly and enter The reaction was carried out in the first microreactor, the volume of the first microreactor was 3mL, the reaction temperature T1: 20°C, the residence time t1: 1min, and the reaction liquid A was obtained. Reaction solution A and 98wt% chloroester aqueous solution are pumped into the second micro-mixer of micro-reaction device respectively, after fully mixing, enter in the second micro-reactor and react, the volume of the second micro-reactor is 24mL, reaction temperature T2 : 25° C.; residence time t2: 6 min, to obtain reaction solution B. The molar ratio of the reaction is: chloroester: hydrogen peroxide: sodium hydroxide=1: 0.6: 1.2. The inner diameters of the reaction tubes of the first ...
Embodiment 2
[0035] Identical with embodiment 1, difference only is: the volume of the first microreactor is 1.5mL, and the volume of the second microreactor is 24mL, and the reaction residence time t1:0.5min in the first microreactor obtains diperoxide Bis(2-ethylhexyl)carbonate, the reaction yield is 93.2%.
Embodiment 3
[0037] Identical with embodiment 1, difference only is: the volume of the first microreactor is 6mL, and the volume of the second microreactor is 24mL, and the reaction residence time t1:2min in the first microreactor obtains dicarbonate peroxide (2-Ethylhexyl) ester, the reaction yield is 97.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com