A kind of nitrated nitrile rubber and its preparation method and application
A technology of nitrile butadiene rubber and nitrated nitrile, which is applied in the field of nitrated nitrile butadiene rubber and its preparation, to achieve the effects of simple process, short reaction time and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
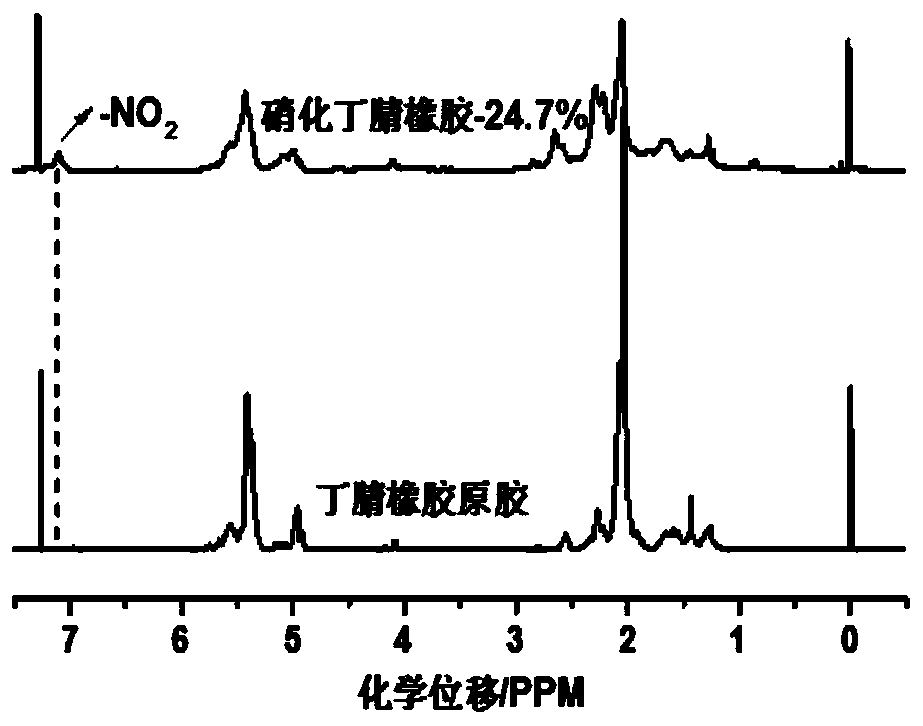
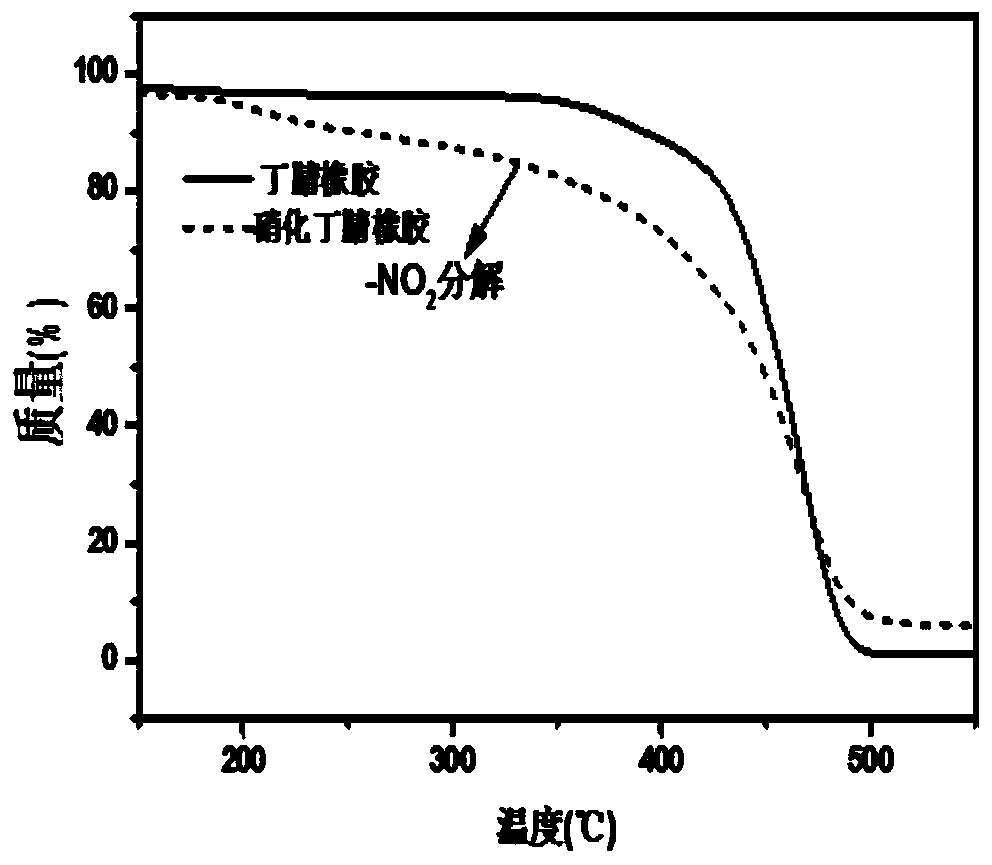
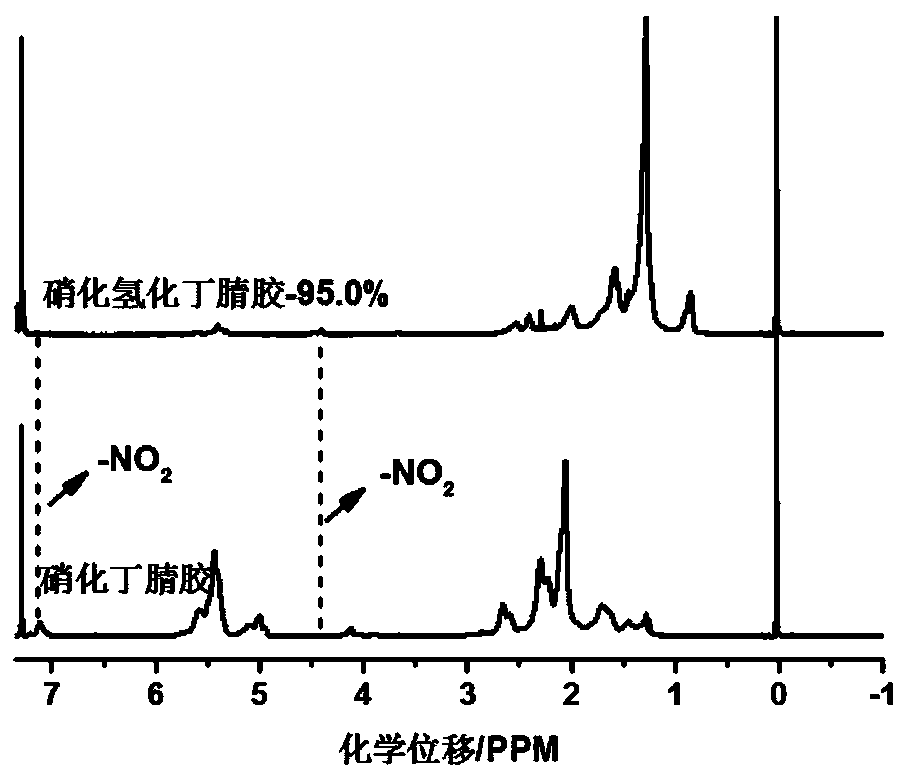
[0041] The present embodiment provides a kind of nitronitrile butadiene rubber and preparation method thereof, and concrete steps are as follows: take chlorobenzene as solvent, take nitrile butadiene rubber as raw material, make nitrile butadiene rubber into nitrile butadiene rubber chlorobenzene that the mass fraction is 7% in advance solution. AgNO 2 and TEMPO were sequentially added to the reaction flask to control the AgNO 2 The consumption of TEMPO is 75% of acrylonitrile-butadiene rubber, and the consumption of TEMPO is 15% of acrylonitrile-butadiene rubber, heats up and carries out nitration reaction, and reaction temperature is 75 ℃, and the reaction time is 12 hours, centrifugal after reaction finishes, and dry 24 hours under 50 ℃ Hour. The hydrogen nuclear magnetic resonance spectrum shows that the nitro group has been successfully inserted on the nitrile rubber and can characterize its degree of nitration, and TG characterizes its thermal stability. The degree of...
Embodiment 2-4
[0044] Embodiment 2-4 provides a kind of nitrated nitrile rubber and preparation method thereof respectively, and concrete condition is as shown in table 1:
[0045] Compared with Example 1, the difference is that the solvents are respectively chlorobenzene, chloroform, toluene and dichloroethane. The degree of nitration of gained nitrile rubber is as shown in table 1.
[0046] The degree of nitration under different solvent conditions in table 1
[0047] solvent Nitrification degree / % Example 2 Chloroform 11.0 Example 3 toluene 13.5 Example 4 Dichloroethane 10.3
Embodiment 5-10
[0049] Embodiment 5-10 provides a kind of nitrated nitrile rubber and preparation method thereof respectively, and specific conditions are as shown in table 2:
[0050] Compared with Example 1, the difference is that AgNO 2 The dosages are respectively 10%, 20%, 30%, 50%, 70%, 90% of the mass of the dry nitrile rubber. The degree of nitration of gained nitrile rubber is as shown in table 2.
[0051] Table 2 Different AgNO 2 The degree of nitrification at the dosage
[0052] AgNO 2 Amount / %
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com