Method for preparing quercetin glycoside
A technology of quercetin glycosides and quercetin, applied in the field of enzyme catalysis, can solve the problems of few species, difficult separation and purification, unfavorable industrial production and application, and achieve high water solubility and overcome low water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of UDP-glucose / N-acetyl-D-glucosamine / UDP-mannose
[0039] Add 21.6mg of glucose, N-acetyl-D-glucosamine and UDP-mannose to a 1.5ml centrifuge tube, add 30.5mgATP, 250ul pH8.0 1M Tris-HCl buffer and 5ul 1M magnesium chloride, 550ul to Ionized water, after mixing, add 200ul 3.9g / L NAHK enzyme, and react overnight at its suitable temperature of 30°C.
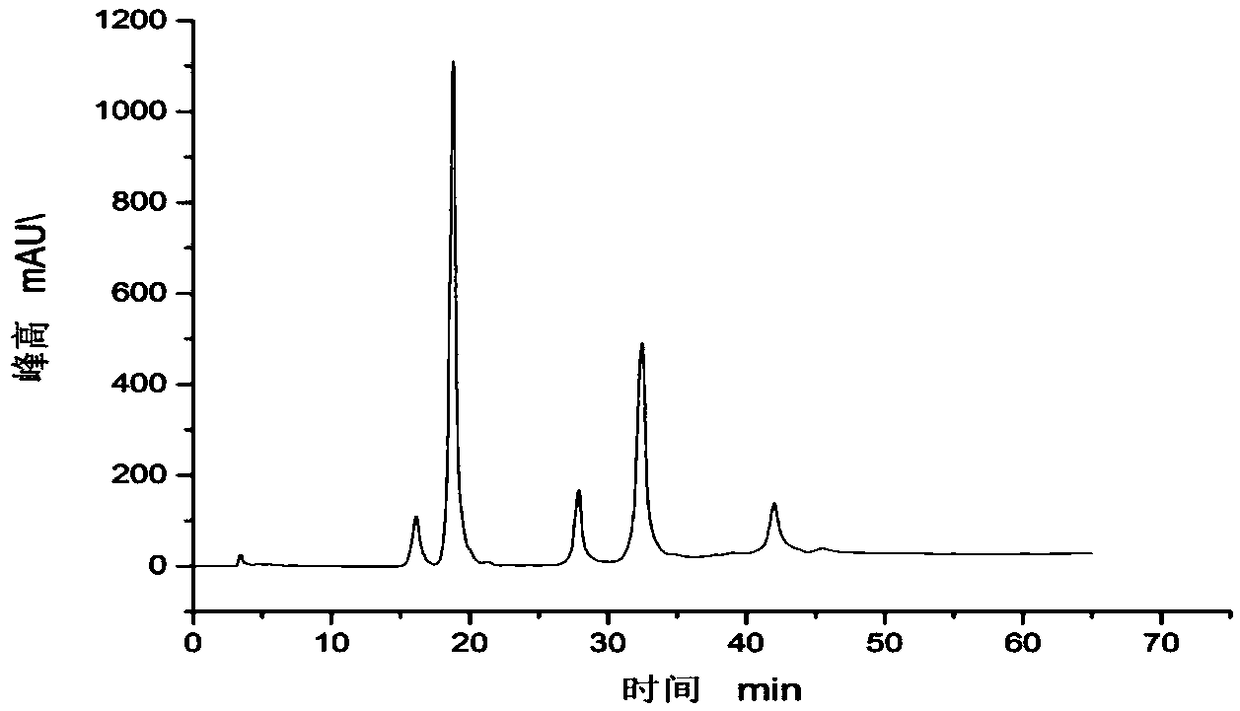
[0040] Add 21mg UTP to the above reaction liquid respectively, add 0.42ul DTT and 2.8ul 1M magnesium chloride, after mixing, add 200ul 9.7g / L Glmu enzyme and 20ul 1.5g / LPPA enzyme, and react at the suitable temperature of 30℃ for more than 5h . After the reaction solution is filtered with a microporous membrane of 0.22uM aperture, carry out high performance liquid chromatography analysis, can detect UDP-glucose, N-acetyl-D-glucosamine and UDP-mannose respectively (such as figure 1 , 2 , 3 shown).
Embodiment 2
[0041] Embodiment 2: Preparation of quercetin glycoside
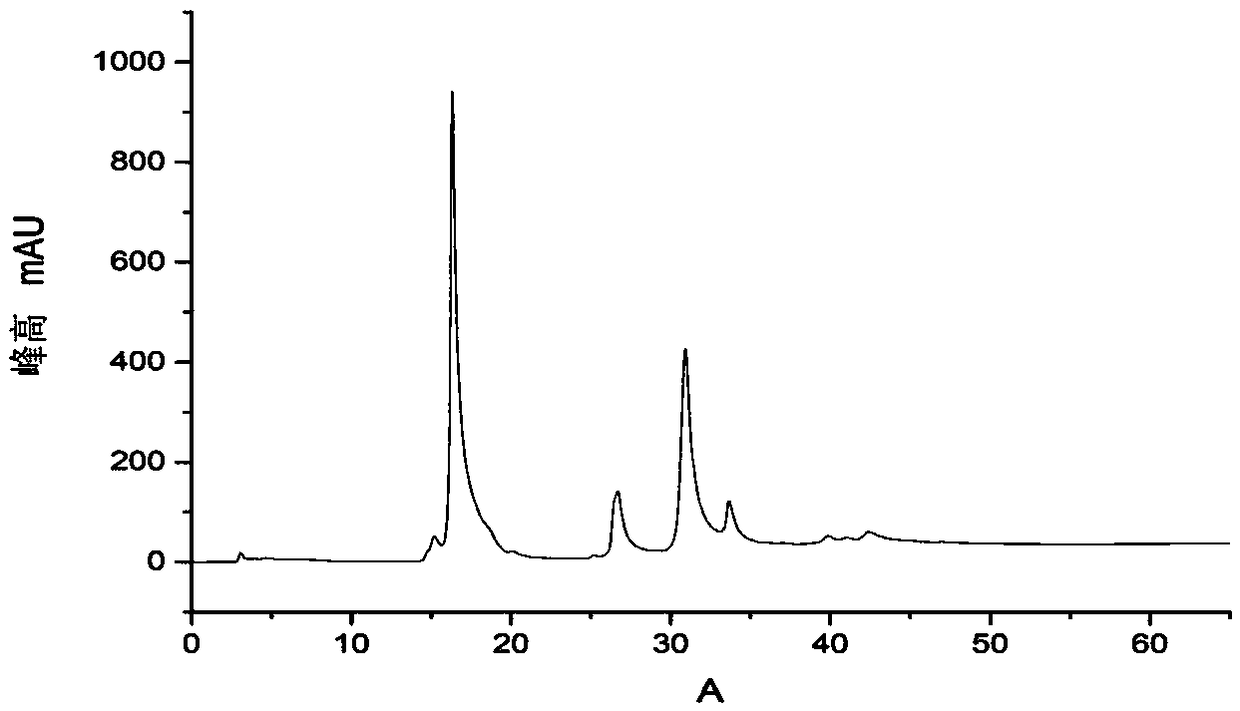
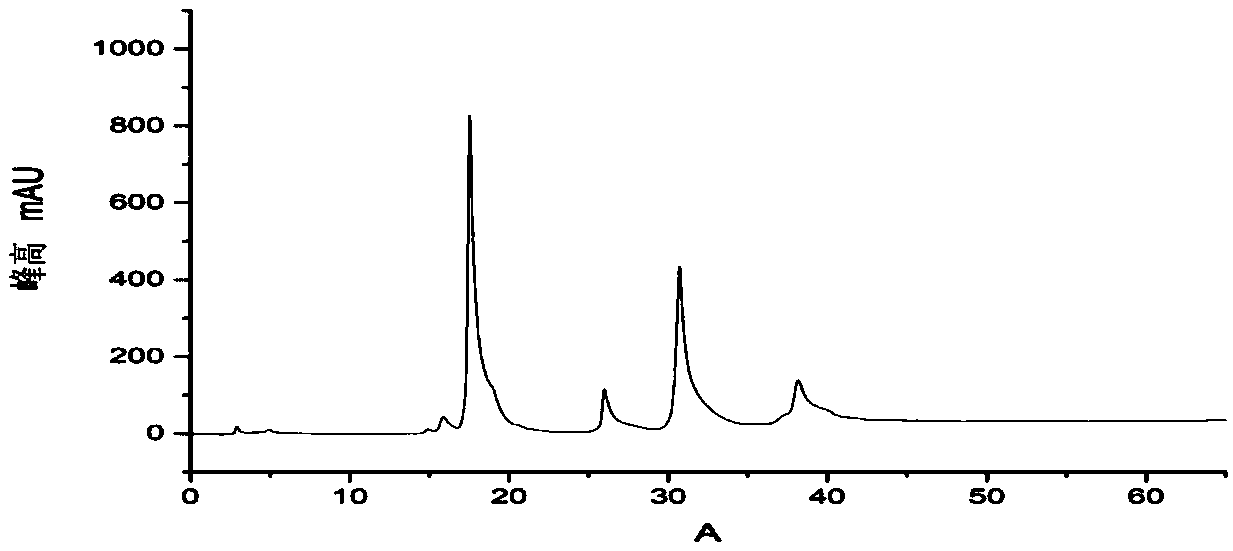
[0042] Add 4ml 20mM quercetin and 2ml 10mM UDP-glucose, UDP-N acetyl-D-glucosamine and UDP-mannose to a 50ml Erlenmeyer flask, then add 5ml 50mM Tris-HCl pH8.0 Buffer solution and 2.5ml 50mM magnesium chloride, 8.5ml deionized water, after mixing, add the glycosyltransferase (its nucleotide sequence as shown in SEQ ID NO.1) in 15ml0.15g / L grapefruit, at its most The reaction was carried out at a suitable catalytic temperature of 35°C for 16 hours; the reaction solution was filtered through a microporous membrane with a pore size of 0.22uM, and analyzed by high performance liquid chromatography. The results were as follows: Figure 4 , 5 , as shown in 6, Figure 4 with Figure 5 It shows that UDP-glucose and UDP-N acetyl-D-glucosamine have reacted completely, and only a single quercetin glycoside product has been detected, indicating that the product catalyzed by the glycosyltransferase in grapefruit is a single produ...
Embodiment 3
[0045] Embodiment 3: the preparation of quercetin glycoside
[0046] Add 1ml 20mM quercetin and 0.5ml 10mM UDP-glucose / UDP-N acetyl-D-glucosamine to a 50ml Erlenmeyer flask, then add 5ml 50mM Tris-HCl buffer solution of pH9.0 and 2.5ml 50mM magnesium chloride, 8.5 ml of deionized water, after mixing, add 15ml of 0.15g / L glycosyltransferase in grapefruit, and react at the optimum catalytic temperature of 30°C for 16h; High-performance liquid chromatography analysis can detect the formation of quercetin glycosides.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com