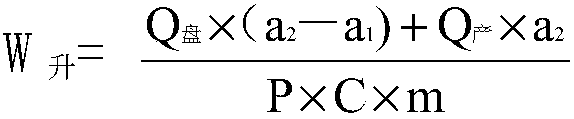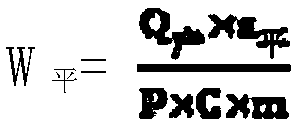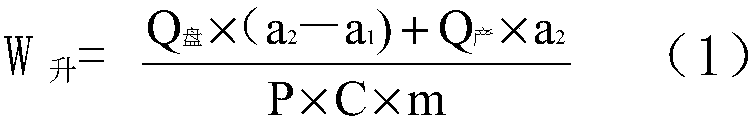Preparation method of aluminum-chromium alloy
A technology of aluminum-chromium alloy and chromium content, applied in the direction of electrodes, electrolysis process, electrolysis components, etc., can solve the problems of flue gas poisoning to human body, low yield of chromium, component segregation, etc., and achieve high utilization rate of chromium raw materials, reduce The effect of high production cost and product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: A method for preparing an aluminum-chromium alloy with a chromium content of 1% by mass, which is prepared using an aluminum electrolytic cell. The electrolytic cell used in this embodiment is a prebaked anode aluminum electrolytic cell, and the anode used is a carbon anode , the cathode is a graphite cathode, the electrolytic cell is a cradle-shaped cell, and the series current is 200KA; the oxide of chromium in the present embodiment is Cr 2 o 3, and its purity is 99%. The technological conditions of the electrolytic cell are: the average voltage is 3.642V, and the electrolysis temperature is 933°C.
[0033] Its preparation method specifically comprises the following steps:
[0034] Step 1: In the aluminum electrolytic cell being produced, measure nNaF · AlF in the electrolyte of the aluminum electrolytic cell 3 , n is a constant from 2.1 to 2.9; KF; CaF 2 ; MgF 2 ;LiF;AL 2 o 3 The mass percentage content, wherein KF is 0.8%; CaF 2 5.1%; MgF 2 2.1%...
Embodiment 2
[0073] Example 2: A method for preparing an aluminum-chromium alloy with a chromium content of 1% by mass, which is prepared using an aluminum electrolytic cell. The electrolytic cell used in this embodiment is a prebaked anode aluminum electrolytic cell, and the anode used is a carbon anode , the cathode is a graphite cathode, the electrolytic cell is a cradle-shaped cell, and the series current is 200KA; the oxide of chromium in the present embodiment is Cr 2 o 3 , and its purity is 99%. The technological conditions of the electrolytic cell are: the average voltage is 3.642V, and the electrolysis temperature is 933°C.
[0074] Its preparation method specifically comprises the following steps:
[0075] Step 1: In the aluminum electrolytic cell being produced, measure nNaF · AlF in the electrolyte of the aluminum electrolytic cell 3 ;KF;CaF 2 ; MgF 2 ;LiF;AL 2 o 3 The mass percentage content, wherein KF is 4.1%; CaF 2 2.1%; MgF 2 4.8%; LiF 2.5%; AL 2 o 3 2.2%; the b...
Embodiment 3
[0078] Example 3: A method for preparing an aluminum-chromium alloy with a chromium content of 1% by mass, using an aluminum electrolytic cell for preparation. The electrolytic cell used in this embodiment is a prebaked anode aluminum electrolytic cell, and the anode used is a carbon anode , the cathode is a graphite cathode, the electrolytic cell is a cradle-shaped cell, and the series current is 200KA; the oxide of chromium in the present embodiment is Cr 2 o 3 , and its purity is 99%. The technological conditions of the electrolytic cell are: the average voltage is 4.028V, and the electrolysis temperature is 948°C.
[0079] Concrete processing step is identical with embodiment 1.
[0080] The aluminum-chromium alloy prepared by electrolysis in this embodiment has a chromium content deviation of less than 0.01%, and the composition is uniform; the raw material utilization rate of the actual chromium oxide reaches 93.1%; the current efficiency is 91.2%; a single electrolyti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com