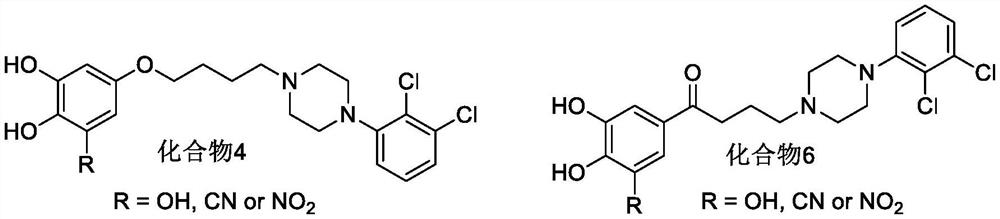
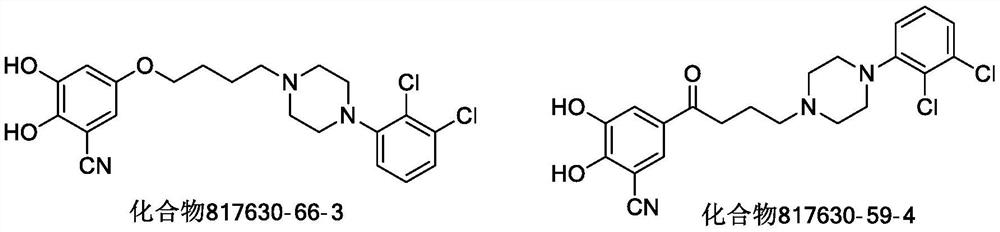
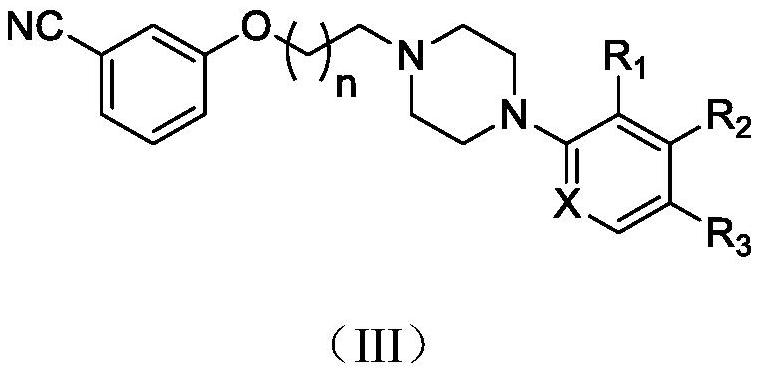
3-Cyanophenoxyalkylarylpiperazine derivatives and their application in the preparation of medicines
A technology of cyanophenoxyalkylaryl and derivatives, applied in the field of 3-cyanophenoxyalkylarylpiperazine derivatives, which can solve the problems of difficult treatment and high muscle tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Preparation of 3-(3-(4-(2,3-dichlorophenyl)piperazin-1-yl)propoxy)benzonitrile (Ⅲ-1) hydrochloride and hydrobromide
[0094] 0.95g (8.0mmol, 1.0eq) of m-cyanophenol, 2.46g (8.0mmol, 1.0eq) of 1-(3-chloropropyl)-4-(2,3-dichlorophenyl)piperazine, K 2 CO 3 4.42g (32mmol, 4.0eq), 1.33g (8.0mmol, 1.0eq) of potassium iodide, dissolved in 50ml of acetonitrile, heated to reflux, stirred and reacted for 5h, stopped heating, evaporated under reduced pressure to remove the solvent, added 50ml of water and 50ml of ethyl acetate each, and allowed to stand The layers were separated, the aqueous phase was extracted three times with 20 ml of ethyl acetate, the organic phases were combined, washed with saturated brine, and the layers were separated. The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. After purification by column chromatography, a pale yellow oil was obtained. 30 ml of ethyl acetate was added to dissolve, and 5M HCl / E...
Embodiment 2
[0101] Preparation of 3-(3-(4-(2,3-dimethylphenyl)piperazin-1-yl)propoxy)benzonitrile (Ⅲ-2) hydrochloride
[0102] 0.95g (8.0mmol, 1.0eq) of m-cyanophenol, 2.13g (8.0mmol, 1.0eq) of 1-(3-chloropropyl)-4-(2,3-dimethylphenyl)piperazine, K 2 CO 3 4.42g (32mmol, 4.0eq), potassium iodide 1.33g (8.0mmol, 1.0eq) were dissolved in 50ml of acetonitrile, the temperature was refluxed, and the reaction was stirred for 5h. The solvent was distilled off under reduced pressure, 50 ml of water and 50 ml of ethyl acetate were added, and the layers were separated. The aqueous phase was extracted three times with 20 ml of ethyl acetate. The organic phases were combined, washed with saturated brine, and separated. The organic phase was washed with anhydrous sodium sulfate. Dry, filter, concentrate the filtrate, and purify by column chromatography to obtain a pale yellow oil, which is dissolved in 30 ml of ethyl acetate, and 5M HCl / EtOAc is added dropwise to adjust pH<3. The solid is precipitat...
Embodiment 3
[0106] Preparation of 3-(2-(4-(2,3-dichlorophenyl)piperazin-1-yl)ethoxy)benzonitrile (Ⅲ-3) hydrochloride
[0107] 0.95g (8.0mmol, 1.0eq) of m-cyanophenol, 2.35g (8.0mmol, 1.0eq) of 1-(3-chloroethyl)-4-(2,3-dichlorophenyl)piperazine, K 2 CO 3 4.42g (32mmol, 4.0eq), 1.33g (8.0mmol, 1.0eq) of potassium iodide, dissolved in 50ml of acetonitrile, heated to reflux, stirred and reacted for 5h, stopped heating, evaporated under reduced pressure to remove the solvent, added 50ml of water and 50ml of ethyl acetate each, and allowed to stand The layers were separated, the aqueous phase was extracted three times with 20 ml of ethyl acetate, the organic phases were combined, washed with saturated brine, and the layers were separated. The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated. After purification by column chromatography, a pale yellow oil was obtained. 30 ml of ethyl acetate was added to dissolve, and 5M HCl / EtOAc was added drop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com