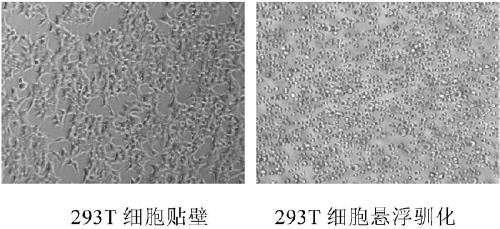
Method for suspension domestication of 293T cells
A cell, full suspension technology, applied in the field of medicine and biology, can solve the problems of contamination of exogenous microorganisms and pathogenic factors, difficulties in downstream purification processes, and biosafety risks, and achieves the goal of being conducive to separation and purification, easy to use, and simple and feasible to operate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
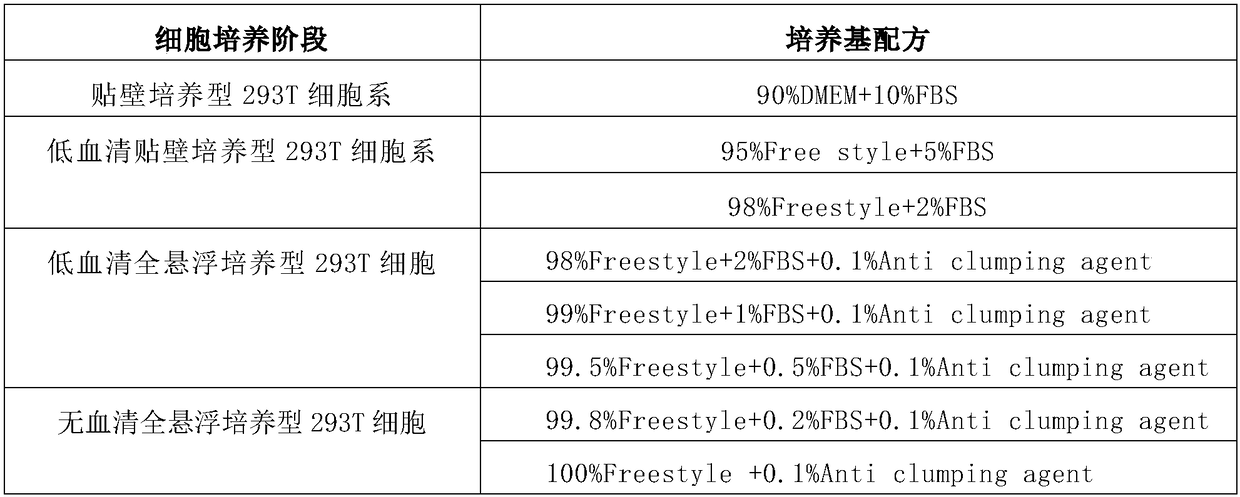
[0018] 1. Reagents used
[0019] 1) Cell line: 293T cell, purchased from ATCC cell bank;
[0020] 2) DMEM culture medium, Corning, article number: 10-013-CVR;
[0021] 3) Free style culture medium, Gibco, article number: 12338-018;
[0022] 4) Trypsin, Gibco, item number: 27250, prepared as 0.25% trypsin solution (EDTA-Na 0.3g / L);
[0023] 5) Anti clumping agent, Invitrogen, article number: 0010057AE.
[0024] 2. The equipment and utensils used
[0025] 1) Shaker, KUHNER, model: ISF1-XC;
[0026] 2) CO2 incubator, Thermo Fisher Scientific Corporation, model: 3111;
[0027] 3) Centrifuge, Eppendorf, model: 5810R;
[0028] 4) Phase contrast inverted microscope, OLYMPUS, OLYMPUS CKX41;
[0029] 5) Cell culture flasks (Corning, T25 article number: 430639, T75 article number: 430641);
[0030] 6) Conical cell flask 150ml, Haimen, Jiangsu, specification: 150ml.
Embodiment 1
[0031] Example 1. Cultivation of adherent culture type 293T cell line
[0032] Select from the company's cell bank, an adherent culture 293T cell that has been tested to be sterile, negative for mycoplasma, and foreign viruses, and cultured with 10% fetal bovine serum (the following serum concentrations are percentages by volume) DMEM, culture conditions: 37 ℃ 5% CO2 incubator culture. The viability of the cells was 98.6% after resuscitation. The cells were cultured for 48 hours to form a compact monolayer, and the cells were subcultured at a ratio of 1:4 to form a compact monolayer at 48 hours, with clear cell edges, flat morphology, and epithelial type.
Embodiment 2
[0033] Example 2. Domestication of low-serum adherent culture 293T cell line
[0034] a) The normal-growing adherent culture 293T cells described in step (1) were cultured in DMEM medium containing 10% fetal bovine serum for three generations each; the standard for each passage is to divide the bottle at a ratio of 1:4, Cultivate in 5% CO2, the cells will form a dense monolayer within 48-72h;
[0035] b) Change the culture medium to Free style medium and add 10% fetal bovine serum to continue culturing for three generations, the passage standard is the same as step a);
[0036] c) The fetal calf serum in the culture medium is reduced to 5%, and the passage ratio is reduced to 1:3 according to the method in step b, and then cultured for three generations;
[0037] d) The fetal bovine serum in the culture medium is reduced to 2%, and the volume percentage of 20% is added to the culture medium. The medium in which the cells have been cultured for 48-72 hours in the previous generation wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com