Aluminum powder surface self-activation method
A self-activation, aluminum powder technology, applied in the field of aluminum powder surface self-activation and aluminum powder surface treatment, can solve the problems of incomplete combustion of aluminum powder, long delay time, slow energy release, etc., to reduce ignition temperature and improve energy density. , the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
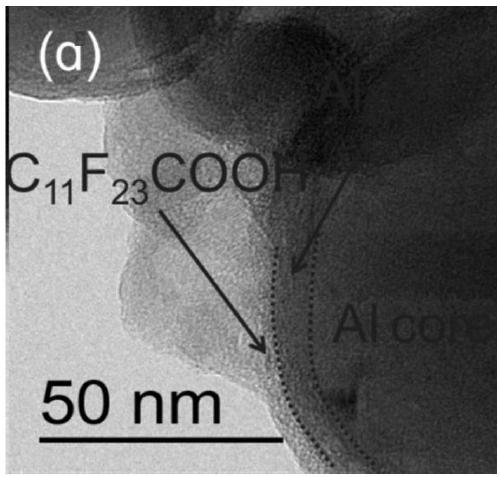
[0028] 0.2g of C 13 f 25 COOH was dissolved in 15 mL of ether solvent to form an organic fluorine solution, and 5 grams of micron aluminum powder (average particle size of 50 microns) was added to the cyclohexane solution, and dispersed by ultrasound for 30 min. Add ether solution to the aluminum powder dispersion at a rate of 5mL / min, stir at 30°C for 2h, centrifuge, wash twice with cyclohexane, and dry in a vacuum oven at 50°C for 5h to obtain self-activated aluminum on the surface of organic fluorine pink. The TEM image of the self-activated aluminum powder on the organic fluorine surface prepared in this example is attached. figure 1 shown, from the attached figure 1 It can be seen that the surface of the aluminum powder has been completely covered by organic fluorine.
Embodiment 2
[0030] 0.3g of C 13 f 25 COOH was dissolved in 15 mL of ether solvent to form a solution of organic fluoride, and 5 grams of nano-aluminum powder (average particle size: 20 nm) was added into the cyclohexane solution, and dispersed by ultrasound for 30 min. Add ether solution to the aluminum powder dispersion at a rate of 5mL / min, stir at 30°C for 2h, centrifuge, wash twice with cyclohexane, and dry in a vacuum oven at 50°C for 5h to obtain self-activated aluminum on the surface of organic fluorine pink.
Embodiment 3
[0032] 0.2g of C 7 f 14 COOH was dissolved in 15 mL of acetonitrile solvent to form a solution of organic fluoride, and 5 grams of nano-aluminum powder (average particle size of 50 nm) was added to the absolute ethanol solution, and dispersed by ultrasound for 30 min. Add ether solution into the aluminum powder dispersion at a rate of 5mL / min, stir at 50°C for 2h, centrifuge, wash twice with absolute ethanol to completely evaporate the solvent, move to 50°C for 5h in a vacuum oven to obtain organic fluorine Surface self-activating aluminum powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com