Synthetic method of 4-methoxysalicylic acid
A technology of methoxysalicylic acid and a synthesis method, which is applied in chemical instruments and methods, separation/purification of carboxylic acid compounds, preparation of carboxylate salts, etc., can solve the problems of complex production process, many industrial waste water, heavy pollution and the like , to achieve the effect of high purity, less waste water and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
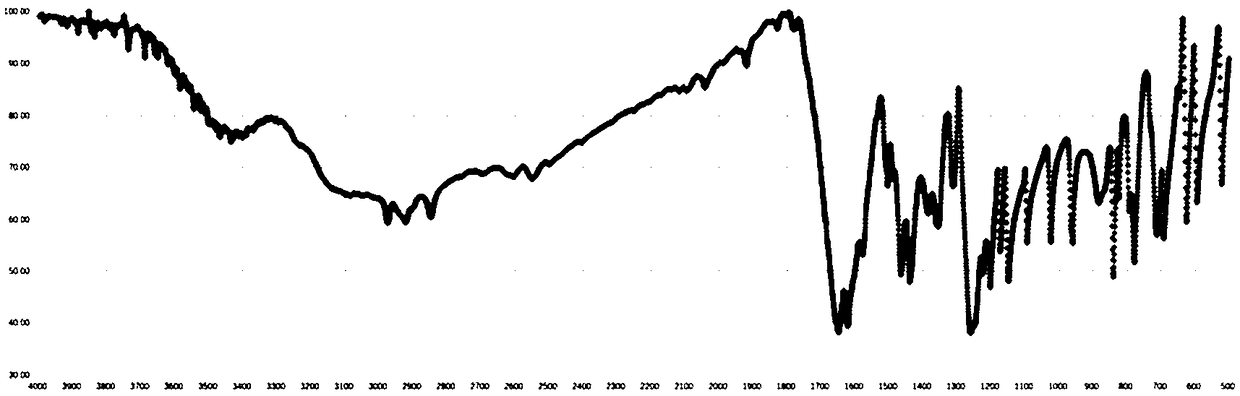
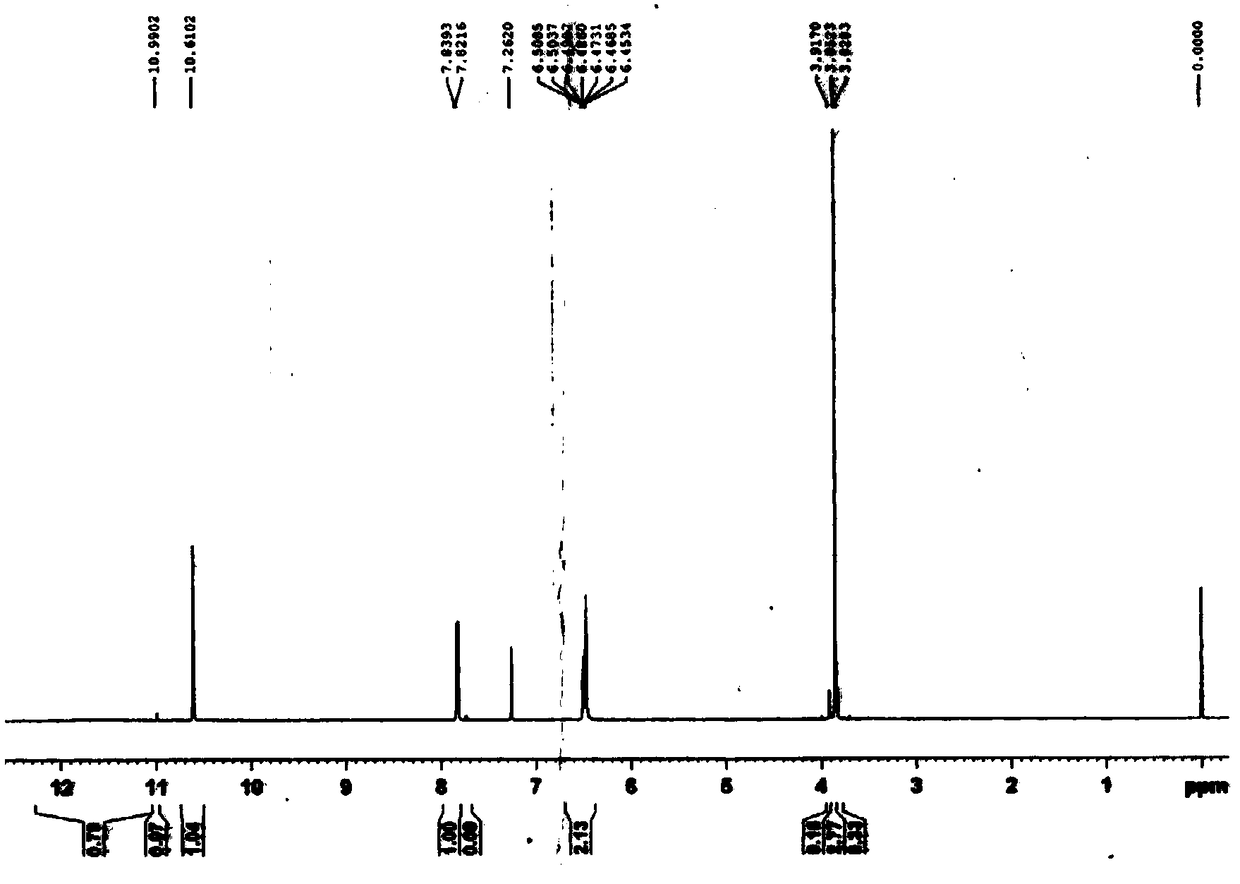
Image
Examples
Embodiment 1
[0022] The specific process of the preparation of 4-methoxy potassium salicylate in the present embodiment is as follows:
[0023] (1) Add 1.62g (0.03mol) sodium methoxide and 9.7ml (0.24mol) methanol solution into a 250ml four-neck flask, slowly add 2.31g (0.015mol) of 2,4-dihydroxybenzoic acid into sodium methoxide -In the methanol mixture, slowly add 1.86 g (0.01 mo) of methyl p-toluenesulfonate dropwise, the temperature is controlled below 30°C, after the dropwise addition is completed, the temperature is raised to 60°C, and stirred for 2h.
[0024] (2) Change the device into an atmospheric distillation device to remove most of the methanol to obtain a reaction mixture. While stirring, the reaction mixture was quickly poured into the hydrochloric acid ice-water mixture, a large amount of white solid matter was precipitated, filtered with suction, rinsed with warm water 2-3 times, and dried to obtain 2 g of 4-methoxysalicylic acid.
[0025] (3) Spread 2-5cm of silicon diox...
Embodiment 2
[0029] The specific process of the preparation of 4-methoxysalicylic acid in the present embodiment is as follows:
[0030] (1) Add 28.13g (0.52mol) of sodium methoxide and 167ml (4.12mol) of methanol into a 500ml four-necked flask, and slowly add 39.9g (0.26mol) of 2,4-dihydroxybenzoic acid into sodium methoxide- To the methanol mixture, 132.29 g (0.71 mol) of methyl p-toluenesulfonate was slowly added dropwise, and the temperature was controlled below 30°C. After the dropwise addition, the temperature was raised to 60°C and stirred for 2 hours.
[0031] (2) Change the device into an atmospheric distillation device to remove most of the methanol to obtain a reaction mixture. While stirring, the reaction mixture was quickly poured into the hydrochloric acid ice-water mixture, a large amount of white solid matter was precipitated, filtered by suction, rinsed with warm water 2-3 times, and dried to obtain 14 g of crude 4-methoxysalicylic acid.
[0032] (3) Spread 2~5cm of silic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com