Synthetic method of ethyl 5-bromo-2-methylnicotinate
A technology of methyl nicotinic acid ethyl ester and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of high safety risk, few synthesis reports, and unsuitability for large-scale industrial production, so as to improve stability and avoid column chromatography process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
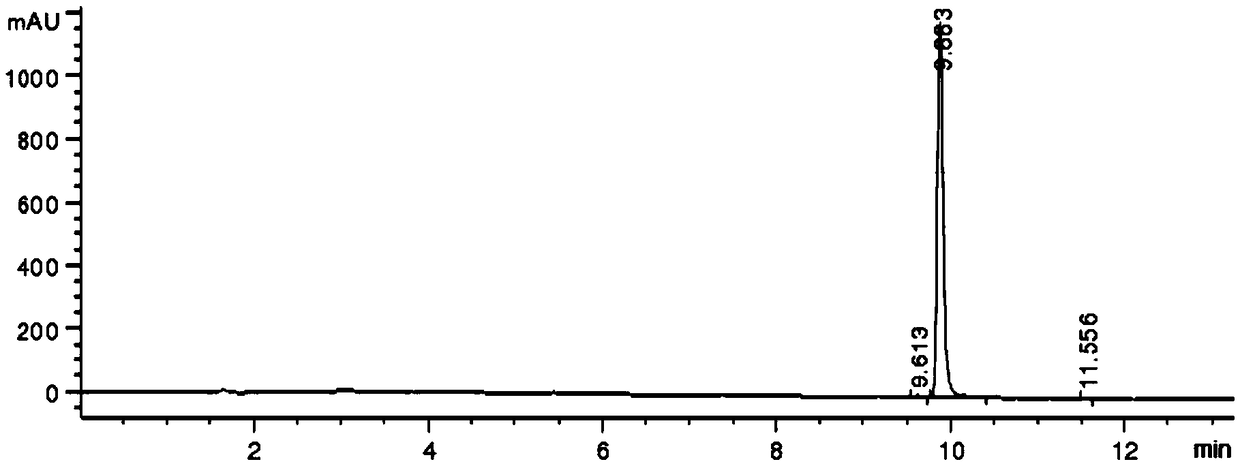
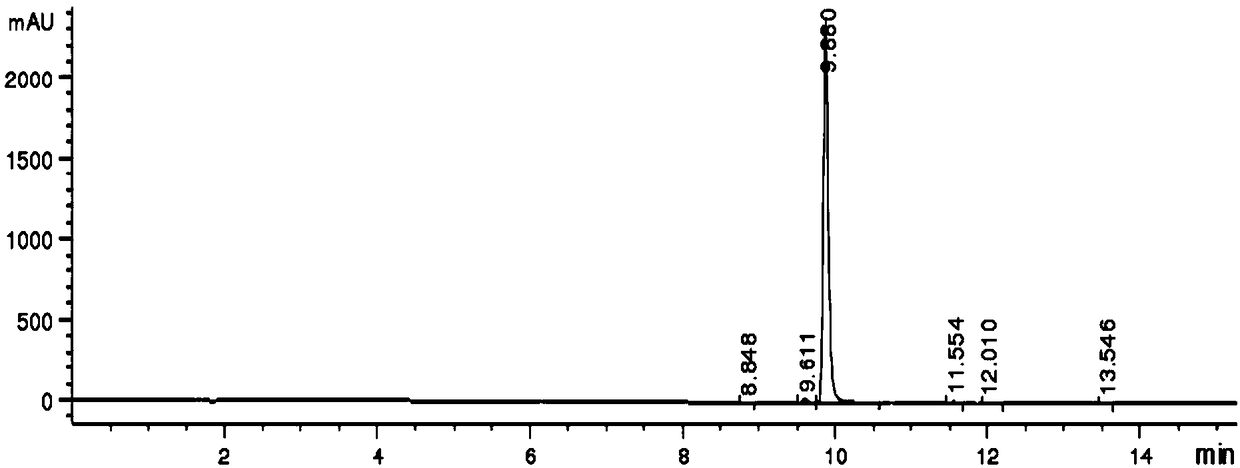
[0037] Add ethyl acetoacetate (7.0 g) and dioxane (140 g) to a 250 mL three-necked flask, lower the temperature of the system to -10° C. in an ice-salt bath, add potassium tert-butoxide (6.4 g), intermediate III (23.8 g) and DABCO (6.1 g) were stirred for 5 hours, ammonium acetate (10.0 g) was added, and the temperature was raised to 60° C. for 16 hours, monitored by TLC until the reaction was complete. The reaction solution was cooled to room temperature, poured into ice water (200 g), added MTBE (50 g), stirred for 1 hour and separated into layers, the aqueous phase MTBE (50 g) was extracted once, and the organic phase was added anhydrous sodium sulfate (20 g). Let dry for 1 hour. Filter, concentrate with a rotary evaporator, dissolve the residue with hot n-heptane (30.0 g), cool down to -20°C, stir for 1 hour, filter, and dry under reduced pressure to obtain ethyl 5-bromo-2-methylnicotinate , 7.8 grams of white solid, yield 60.0%, purity 99.4% (see Table 1).
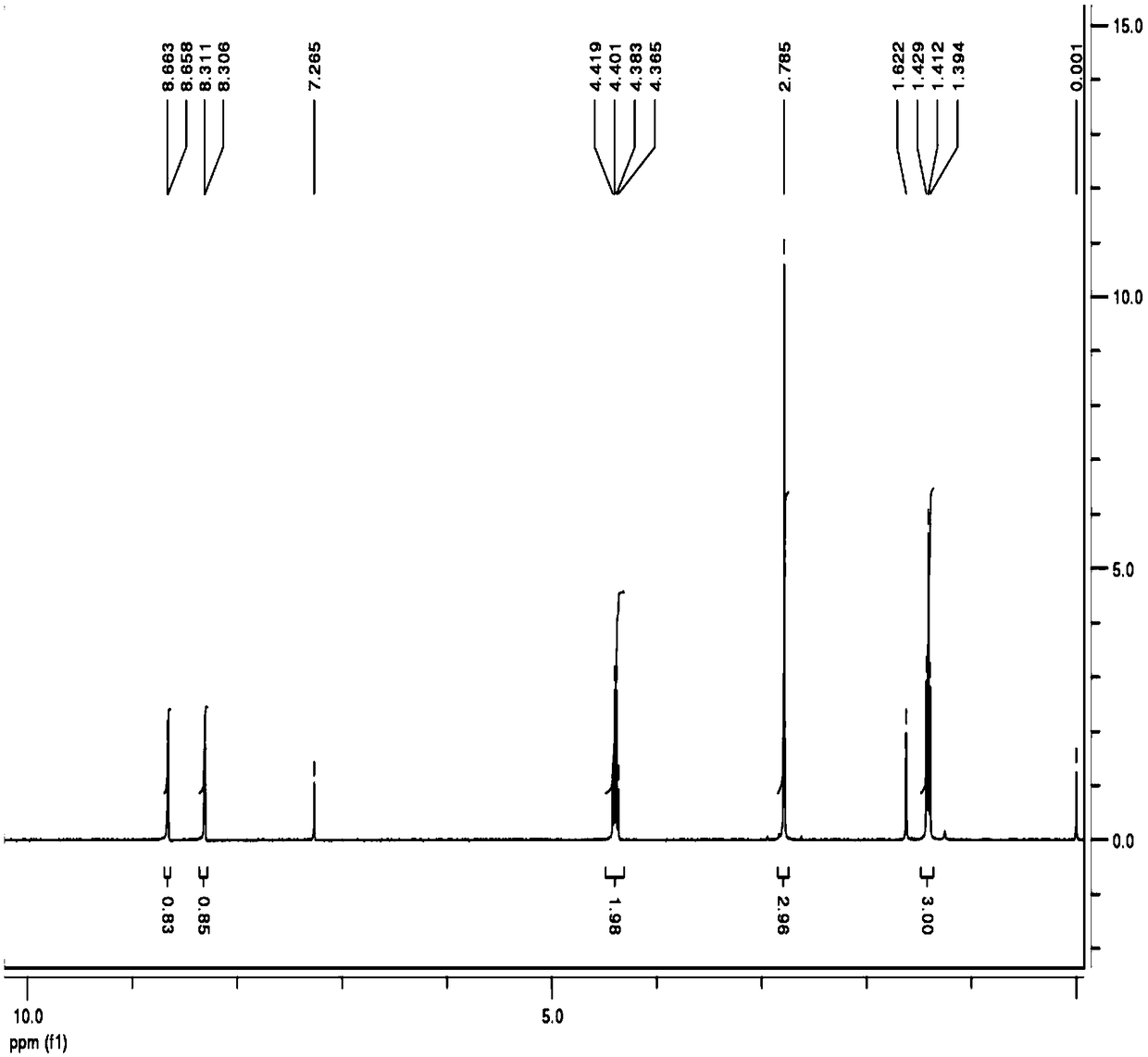
[0038] Its ...
Embodiment 2
[0041] Add ethyl acetoacetate (7.0 g) and 2-methyltetrahydrofuran (70 g) into a 250 mL three-necked flask, lower the temperature of the system to -8° C. in an ice-salt bath, add potassium tert-butoxide (3.5 g), intermediate Compound III (21.0 g) and DABCO (3.5 g) were stirred for 4 hours, ammonium acetate (7.0 g) was added, and the temperature was raised to 55° C. for 15 hours, monitored by TLC until the reaction was complete. The reaction solution was cooled to room temperature, poured into ice water (180 grams), added MTBE (46 grams), stirred for 1 hour and separated into layers, the aqueous phase MTBE (46 grams) was extracted once, and the organic phase was added anhydrous sodium sulfate (20 grams). Let dry for 1 hour. Filtrate, concentrate with a rotary evaporator, dissolve the residue with hot n-heptane (20.0 g), cool to 0°C, stir for 1 hour, filter, and dry under reduced pressure to obtain ethyl 5-bromo-2-methylnicotinate. 6.4 g of white solid, yield 49.0%.
Embodiment 3
[0043] Add ethyl acetoacetate (7.0 g) and tetrahydrofuran (210 g) into a 250 mL three-necked flask, lower the temperature of the system to -6°C under an ice-salt bath, add potassium tert-butoxide (7.0 g), intermediate III (22.5 gram) and DABCO (4.5 grams), stirred for 4 hours, added ammonium acetate (9.0 grams), heated to 57 ° C for 16 hours, TLC monitoring until the end of the reaction. The reaction solution was cooled to room temperature, poured into ice water (180 grams), added MTBE (48 grams), stirred for 1 hour and separated into layers, the aqueous phase MTBE (46 grams) was extracted once, and the organic phase was added anhydrous sodium sulfate (20 grams). Let dry for 1 hour. Filtrate, concentrate with a rotary evaporator, dissolve the residue with hot n-heptane (25.0 g), raise it to 20°C, stir for 1 hour, filter, and dry under reduced pressure to obtain ethyl 5-bromo-2-methylnicotinate. 6.4 g of white solid, yield 49.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com