Near-infrared photothermal activation delayed fluorescent material and preparation method thereof and display device
A thermally activated delayed, near-infrared light technology, applied in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problems of short service life, lack of thermally activated delayed fluorescent materials, and low fluorescence efficiency. Effects of fast reverse intersystem crossing constant, reduction of lowest single-triplet energy level difference, and high photoluminescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
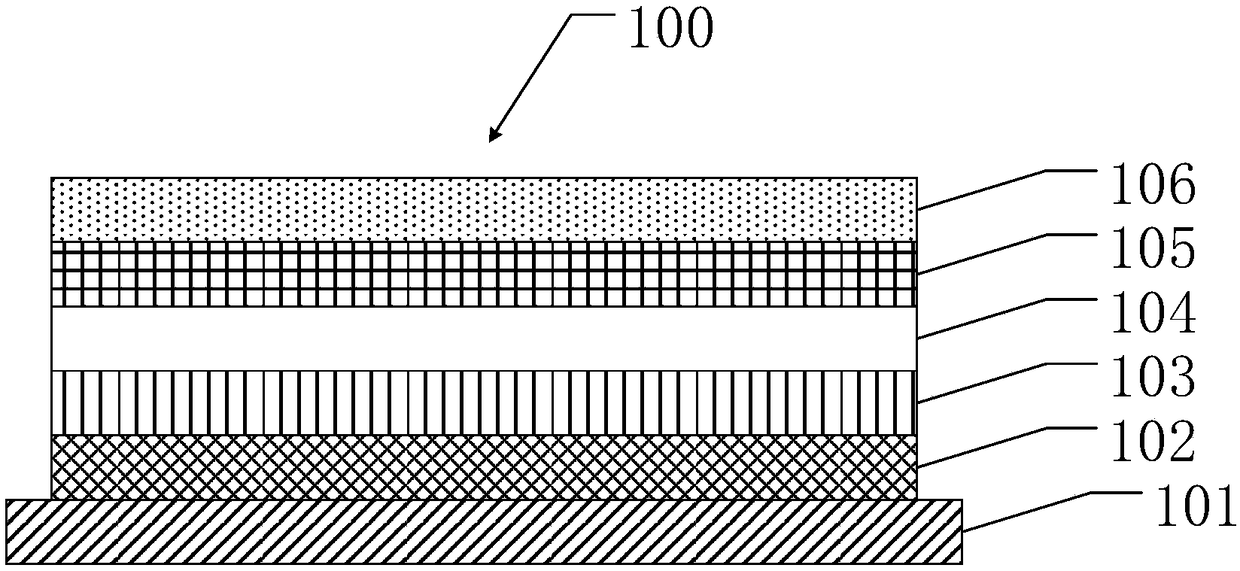
Image
Examples
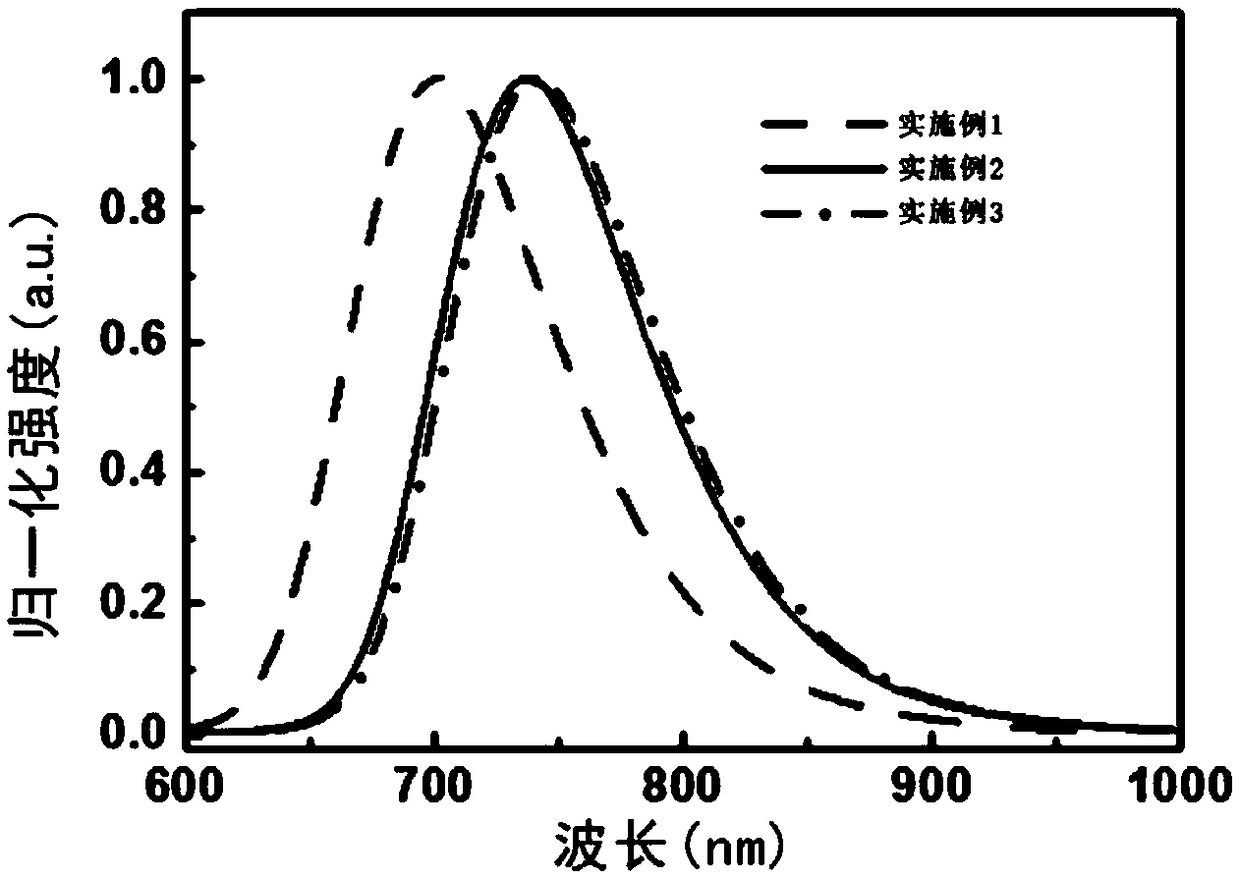
Embodiment 1
[0034] This embodiment is a preferred embodiment.
[0035] The molecular structure of the near-infrared photothermally activated delayed fluorescence material provided in this example is the D-A-D structure generated by the reaction of the electron donor (D) and the electron acceptor (A), and the electron acceptor (A) is in the triplet energy level range The planar electron acceptor (A) with a value of 1.30-1.80, and the electron donor (D) have strong electron donating ability.
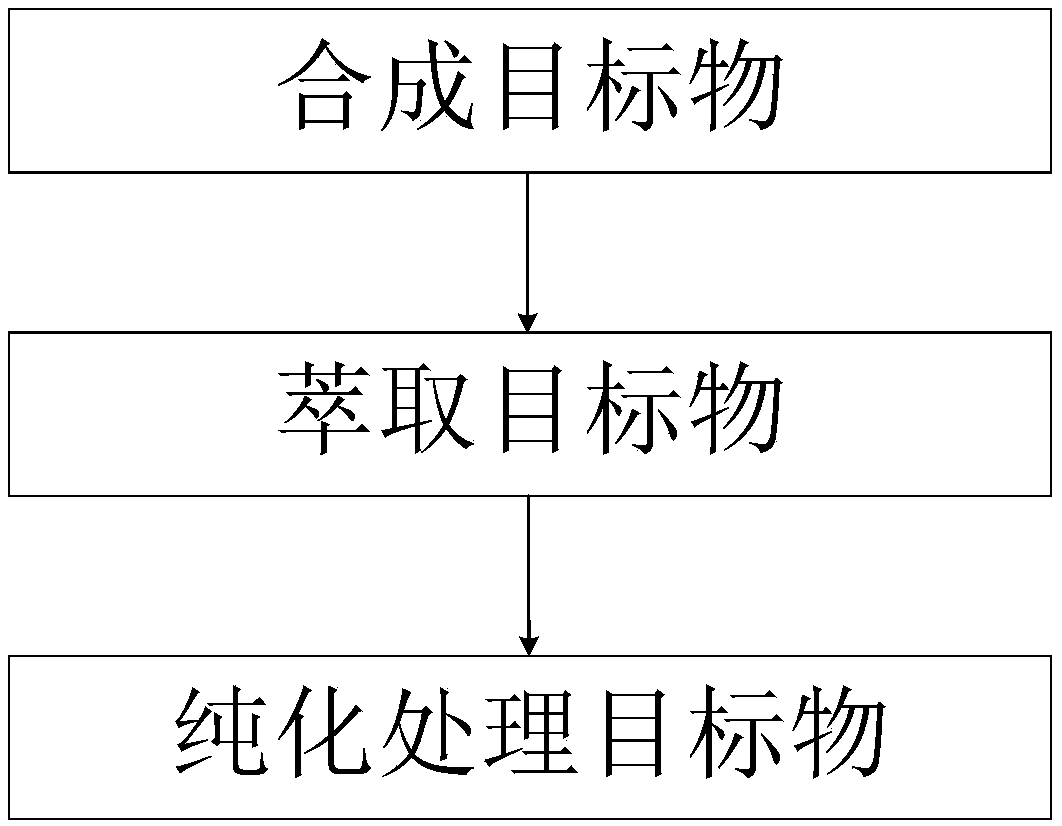
[0036] In this example, the compound with electron acceptor (A) is 2,5-bis(4-bromophenyl)imidazo[4,5-d]imidazole, and the compound with electron donor (D) is 9'9 -Dimethylacridine. The preparation process is as figure 2 As shown, the specific preparation steps are as follows:
[0037] Synthesis of the target object: In a 100mL two-neck flask, add 2,5-bis(4-bromophenyl)imidazo[4,5-d]imidazole (2.12g, 5mmol), 9'9-dimethylacridine Pyridine (2.5g, 12mmol), palladium acetate (90mg, 0.4mmol) and tri-te...
Embodiment 2
[0050] The near-infrared photothermally activated delayed fluorescence material provided in this example adopts a D-A-D structure in which an electron donor (D) and an electron acceptor (A) are combined, and the electron acceptor (A) has a triplet energy level range of 1.30 -1.80 planar electron acceptor (A) and electron donor (D) have strong electron donating ability.
[0051] In this example, the compound with electron acceptor (A) is 2,5-bis(4-bromophenyl)imidazo[4,5-d]imidazole, and the compound with electron donor (D) is phenoxazine . The preparation process is as figure 2 As shown, the specific preparation steps are as follows:
[0052] Synthesis of the target object: In a 100mL two-necked flask, add 2,5-bis(4-bromophenyl)imidazo[4,5-d]imidazole (2.12g, 5mmol), phenoxazine (2.2g, 12mmol ), palladium acetate (90mg, 0.4mmol) and tri-tert-butylphosphine tetrafluoroborate (0.34g, 1.2mmol), then place the reaction vessel in a box filled with argon, then Sodium tert-butox...
Embodiment 3
[0065] The near-infrared photothermally activated delayed fluorescence material provided in this example adopts a D-A-D structure in which an electron donor (D) and an electron acceptor (A) are combined, and the electron acceptor (A) has a triplet energy level range of 1.30 -1.80 planar electron acceptor (A) and electron donor (D) have strong electron donating ability.
[0066] In this example, the compound with electron acceptor (A) is 2,5-bis(4-bromophenyl)imidazo[4,5-d]imidazole, and the compound with electron donor (D) is phenothiazine . The preparation process is as figure 2 As shown, the specific preparation steps are as follows:
[0067] Synthesis of the target object: In a 100mL two-necked flask, add 2,5-bis(4-bromophenyl)imidazo[4,5-d]imidazole (2.12g, 5mmol), phenothiazine (2.2g, 12mmol ), palladium acetate (90mg, 0.4mmol) and tri-tert-butylphosphine tetrafluoroborate (0.34g, 1.2mmol), then place the reaction vessel in a box filled with argon, then Sodium tert-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com