Method for expressing and purifying novel cell reprogramming factor
A technology of cells and expression vectors, applied in the field of expression and purification of new cell reprogramming factors, which can solve the problems of reduced transmembrane activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
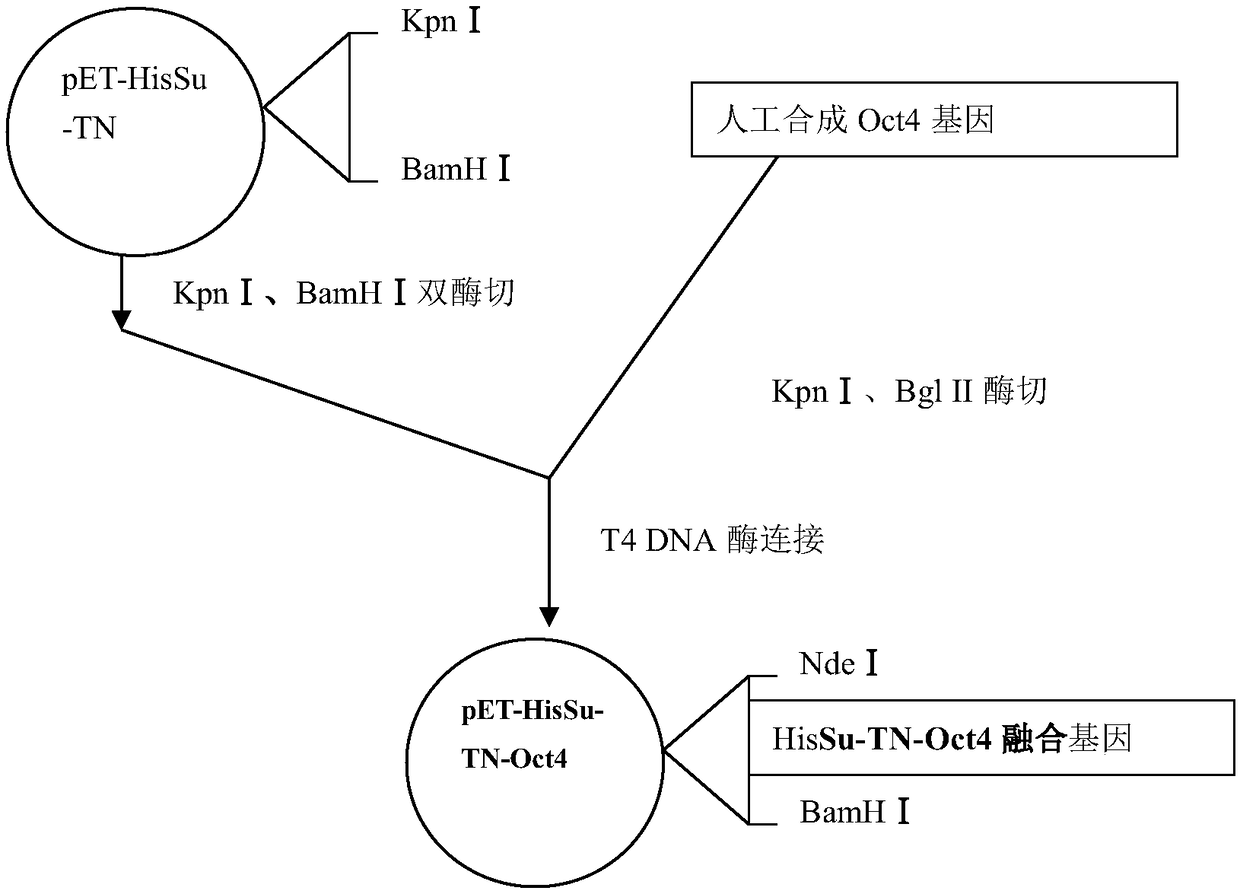
[0026] Example 1 Construction of pET-HisSu-TN-Oct4 fusion protein expression vector
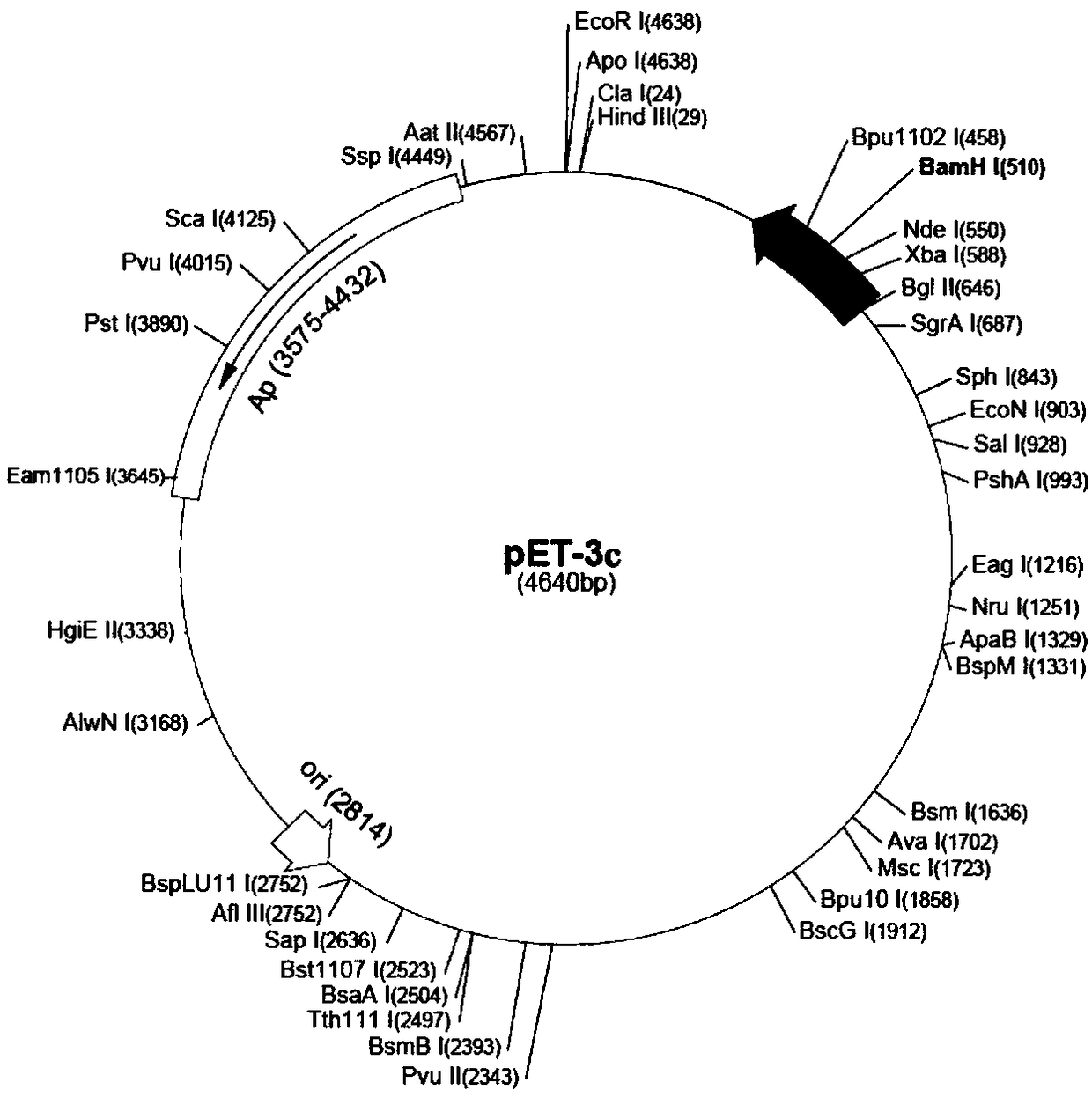
[0027] use as figure 1 The indicated expression vector pET-3c (purchased from Novagen, USA) clones and expresses the HisSu-TN-Oct4 fusion protein of the present invention.
[0028] The pET-3c vector has multiple cloning sites. According to the restriction site of pET-3c vector and the cDNA sequence of Oct4 protein, the following primers were designed:
[0029] OctF:GC GGTACC GCTGGACACCTGGCTTCAG (SEQ ID NO.3); contains Kpn I restriction site (underlined).
[0030] OctR:GA AGATCT TCAGTTTGAATGCATGGGAGAGCCC (SEQ ID NO. 4), containing a Bgl II restriction site (underlined).
[0031] Oct4 cDNA was amplified using TetO-FUW-OSKM plasmid as a template. The PCR reaction conditions were: 1×PCR reaction buffer, 0.5 μM Oct-F, 0.5 μM Oct-R, 1 μg TetO-FUW-OSKM plasmid, 2U DNA polymerase (Fermentas), 50 μM dATP, 50 μM dTTP, 50 μM dCTP, 50μM dGTP, 1.5mM MgCl2, adjust the reaction volume to 50μl with ...
Embodiment 2
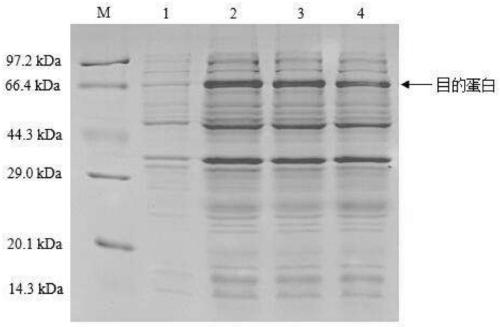
[0036] Example 2 Expression and purification of pET-HisSu-TN-Oct4 expression vector
[0037] The recombinant DNA plasmid pET-HisSu-TN-Oct4 obtained above was transformed into Escherichia coli Rossate (DE3) (purchased from Novagen, USA). The transformants of Escherichia coli Rossate (DE3) containing pET-HisSu-TN-Oct4 were inoculated in LB medium containing 50 μg / ml of ampicillin and chloramphenicol, and cultured overnight at 37°C. Inoculate into LB liquid medium (containing ampicillin and chloramphenicol) with 1% inoculum amount, and culture on a shaker at 37°C until OD 600 0.5 to 0.6. Human isopropyl-β-D-thiogalactopyranoside (IPTG for short) was added to the culture solution to a final concentration of 0.5 mM, induced at 30°C for 4 hours, and the cells were collected by centrifugation. The bacteria were resuspended in 50 mM sodium phosphate buffer (containing 300 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF for short), pH 7.4). Sonicate the cell wall, centrifuge at 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com