Catalyst used for cathode reaction of fuel cell and preparation method thereof
A fuel cell cathode and catalyst technology, applied in battery electrodes, nanotechnology for materials and surface science, circuits, etc., can solve problems such as inconvenience, complex catalyst preparation methods, and large gaps in catalytic performance and stability, and achieve Simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the preparation of metal organic framework
[0033] 1.071g of zinc nitrate hexahydrate and 2.31g of 2-methylimidazole were mixed in an aqueous solution, and after ten minutes of stirring, the material was completely dissolved, and then nucleated growth and precipitation were carried out for 24 hours, and finally the obtained precipitate was Separation and drying are carried out to obtain a metal-organic framework.
Embodiment 2
[0034] Embodiment two, preparation and performance detection of catalyst 1
[0035] Weigh 800 mg of the metal-organic framework prepared in Example 1, mix it with 200, 400 or 600 mg of cobalt nitrate nonahydrate in an aqueous solution, stir for 1 hour, dry the material, and then place the dried material in a nitrogen atmosphere , heated to 700°C, and kept warm for 0.5-3 hours.
[0036]
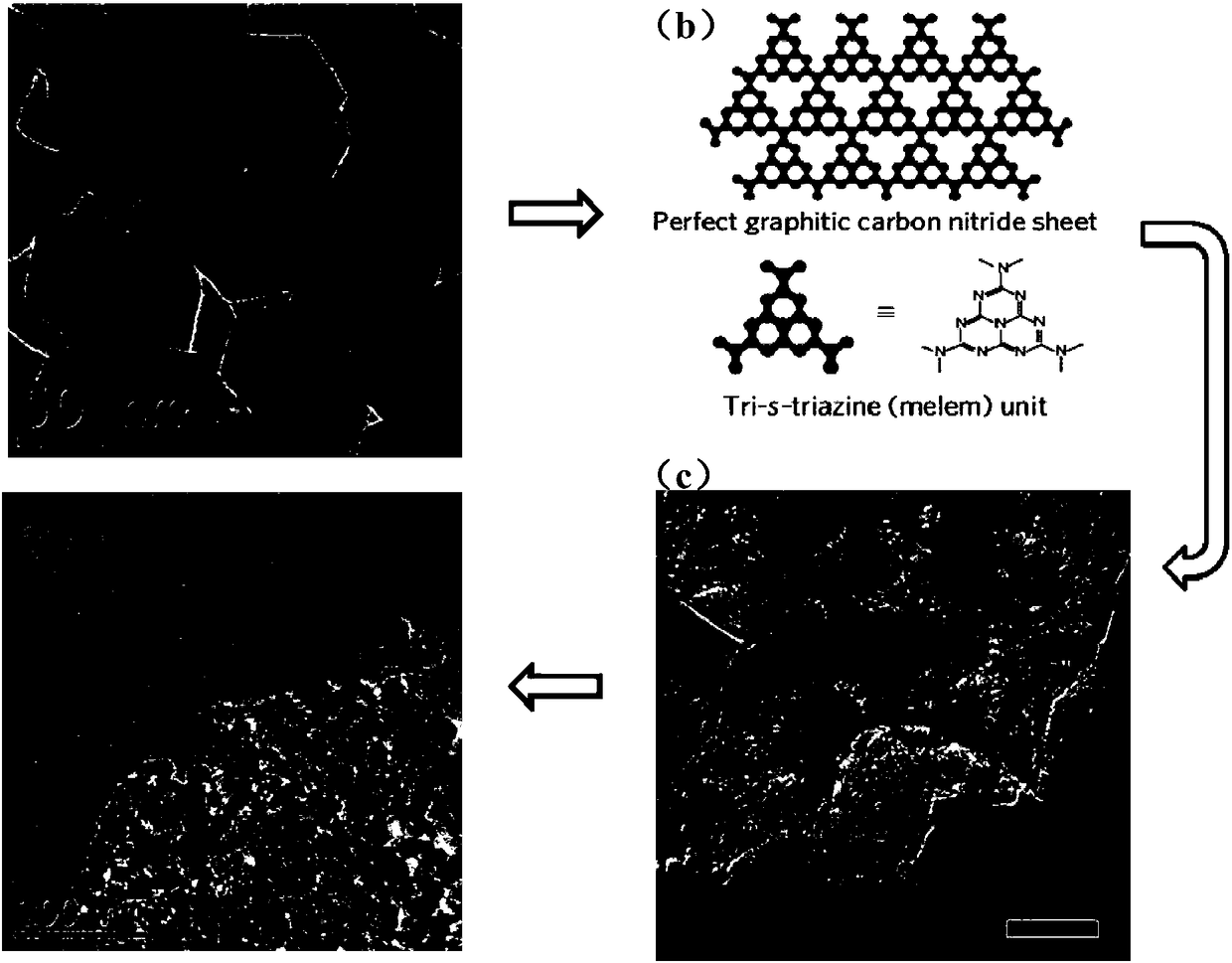
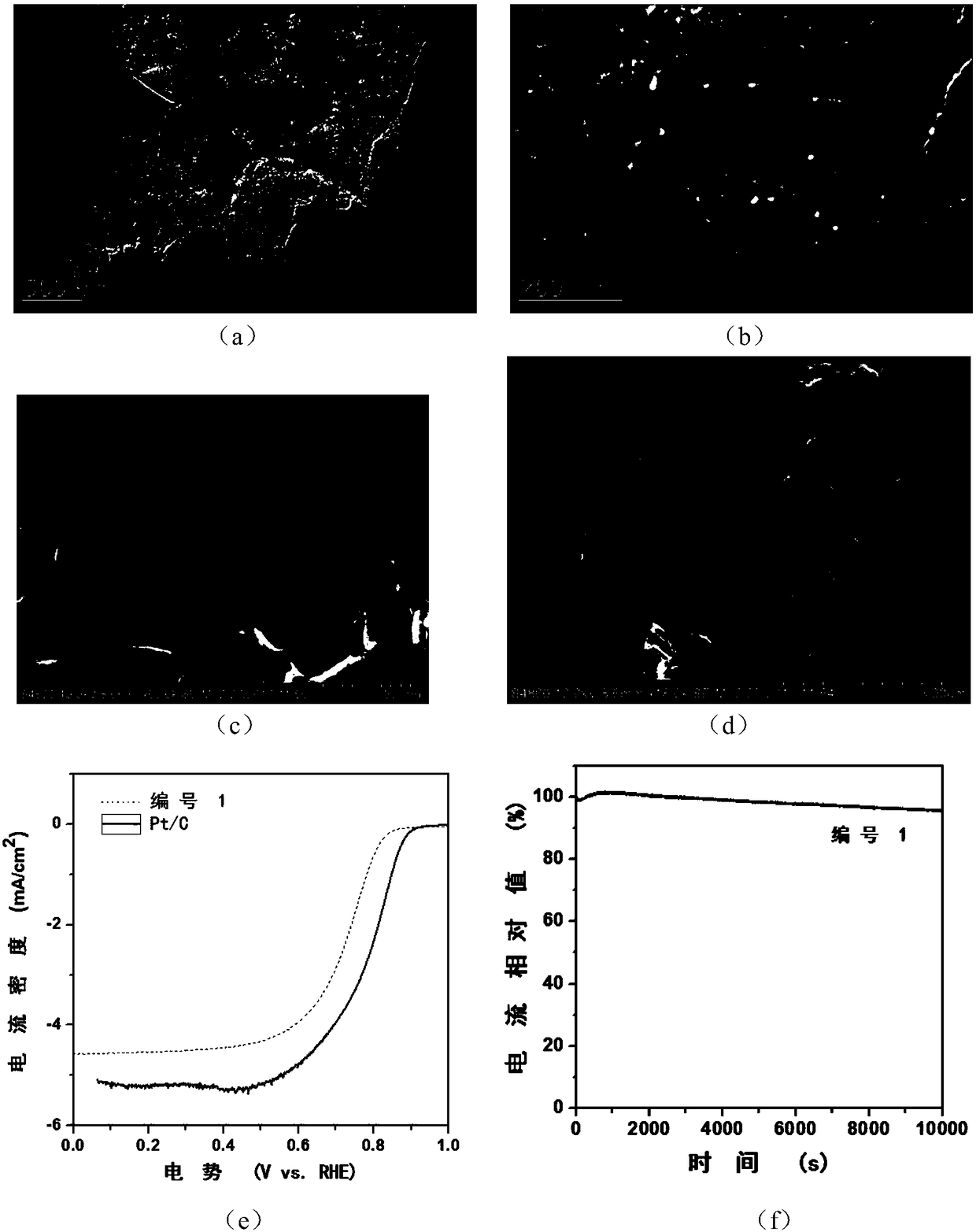
[0037] By comparing the catalysts obtained with different cobalt nitrate additions and different pyrolysis times, it can be concluded that the catalysts have good catalytic performance when pyrolyzed at 700°C, and the catalytic performance of the catalyst material with the addition of 400mg of inorganic salts and heat preservation for 1 hour It is the best, and it is numbered 1, and its catalytic performance is shown in Table 1. The electron micrograph of No. 1 catalyst is as follows image 3As shown in (a) to (d), it can be seen that the main composition of the catalyst is that nanoparti...
Embodiment 3
[0038] Embodiment three, preparation and performance detection of catalyst 2
[0039] Weigh 800 mg of the metal-organic framework prepared in Example 1, mix it with 200, 400 or 600 mg of cobalt nitrate nonahydrate in an aqueous solution, stir for 1 hour, dry the material, and then place the dried material in a nitrogen atmosphere , heated to 800°C, and kept warm for 0.5-3 hours.
[0040]
[0041] By comparing the catalysts obtained with different cobalt nitrate additions and different pyrolysis times, it can be concluded that the catalysts have good catalytic performance when pyrolyzed at 800°C, and the catalytic performance of the catalyst material with the addition of 400mg of inorganic salts and heat preservation for 1 hour It is the best, and it is numbered 2, and its catalytic performance is shown in Table 1. The electron micrograph of No. 2 catalyst is as Figure 4 As shown in (a) to (d), it can be seen that the main composition of the catalyst is that nanoparticles...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com