Amphiphilic polymer prodrug for reducing responsive 7-ethyl-10-hydroxycamptothecine and preparation method thereof
A technology of amphiphilic polymers and hydroxycamptothecin, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations of non-active ingredients, etc., can solve the problem of not being able to release the original drug, and achieve mass production and easy availability of raw materials , the effect of high yield and product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
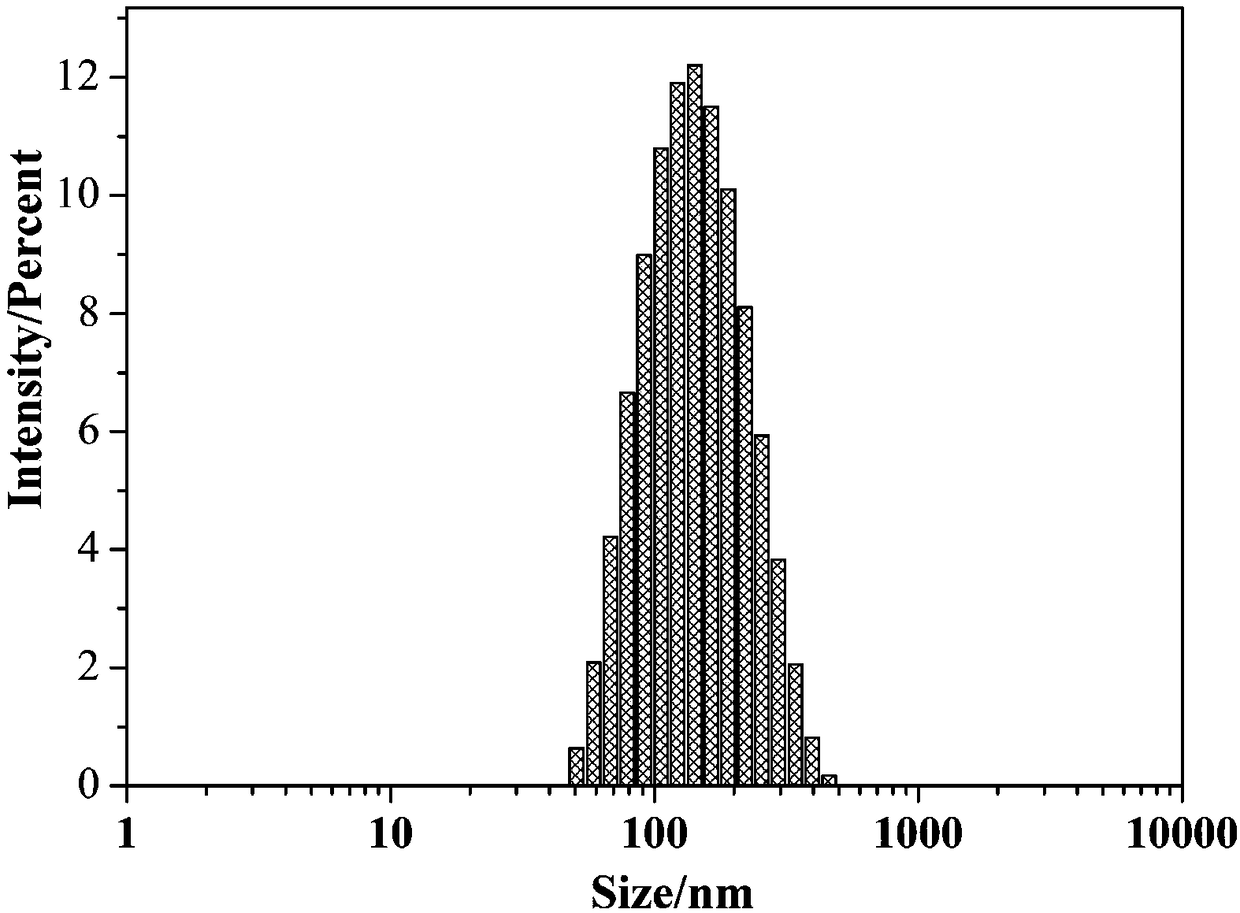
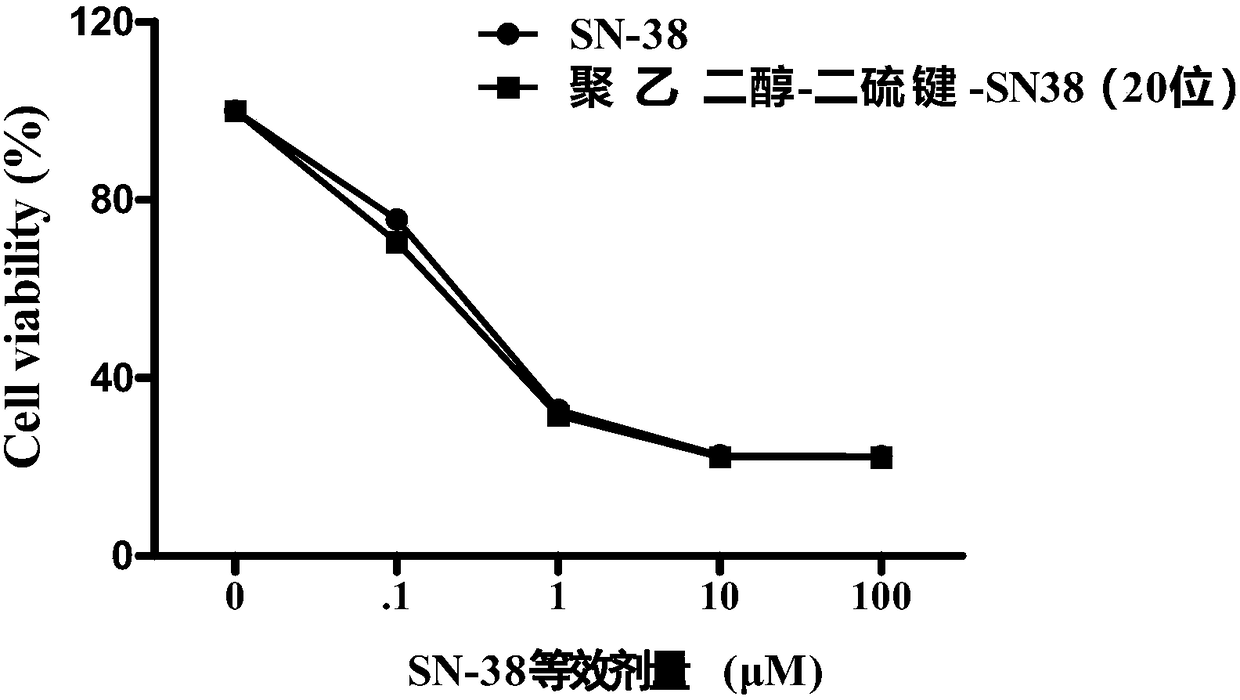
Examples
Embodiment 1
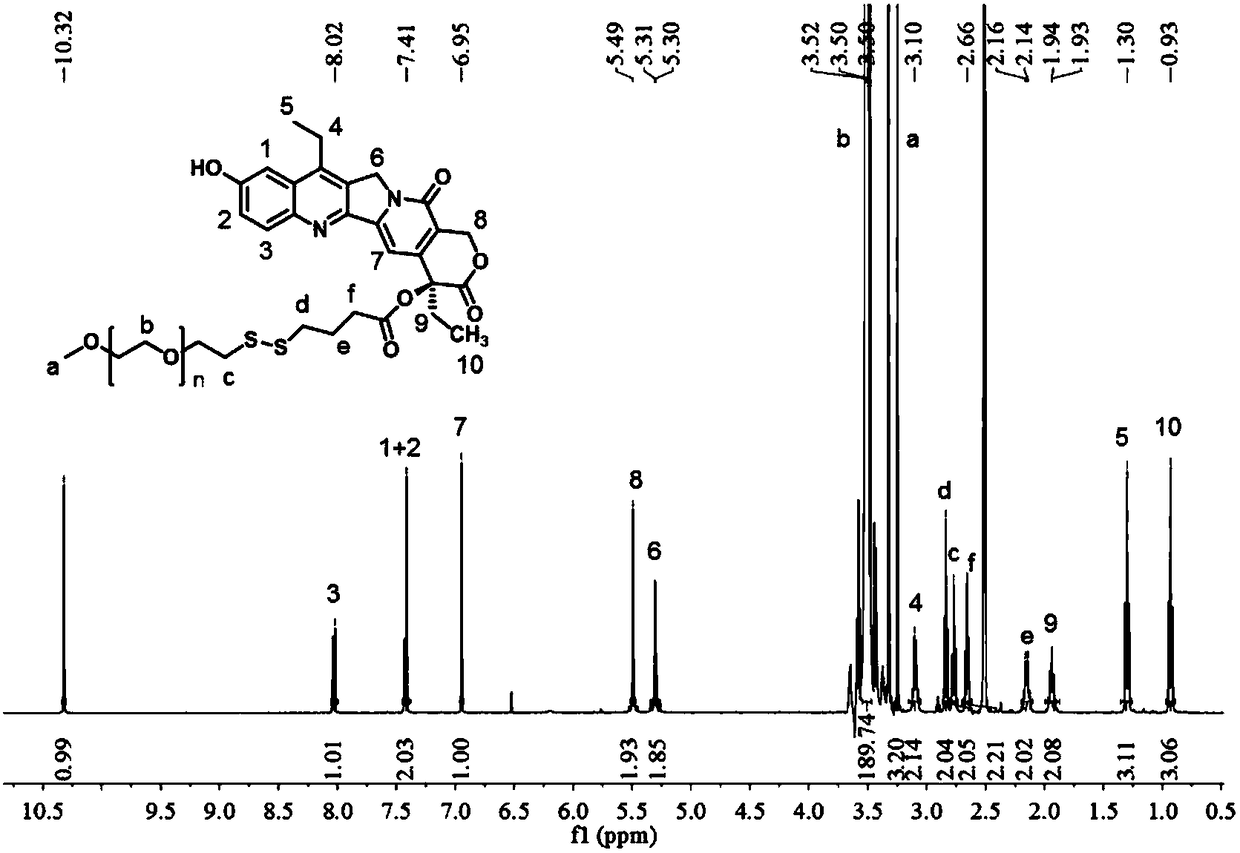
[0040] A reduction-responsive amphiphilic polymer prodrug of 7-ethyl-10-hydroxycamptothecin has a molecular structure as shown below:
[0041]
[0042] Where n is 40-50.
[0043] The preparation method of above-mentioned amphiphilic polymer prodrug is as follows:
[0044](1) Preparation of TBDPS-7-ethyl-10-hydroxycamptothecin:
[0045] Add 7-ethyl-10-hydroxycamptothecin (2.0 g, 5.1 mmol) into 100 mL of dichloromethane, add triethylamine (3.2 mL, 23 mmol) and TBDPSCl (5.3 mL, 20.4 mmol), and reflux overnight. After cooling to room temperature, the organic phase was washed successively with dilute hydrochloric acid, saturated sodium bicarbonate, and saturated brine, separated, dried, and concentrated under reduced pressure. The concentrated solution was dropped into a large amount of n-hexane for precipitation, filtered, and vacuum-dried to obtain 3.0 g of a yellow solid with a yield of 93%. product 1 H NMR and MS tests showed that TBDPS-7-ethyl-10-hydroxycamptothecin wit...
Embodiment 2
[0056] A reduction-responsive amphiphilic polymer prodrug of 7-ethyl-10-hydroxycamptothecin has a molecular structure as shown below:
[0057]
[0058] where 400
[0059] The preparation method of above-mentioned amphiphilic polymer prodrug is as follows:
[0060] (1) (preparation of TMS-7-ethyl-10-hydroxycamptothecin:
[0061] 7-Ethyl-10-hydroxycamptothecin (2.0 g, 5.1 mmol) was added to 100 mL of dichloromethane, triethylamine (3.2 mL, 23 mmol) and (CH 3 ) 3 SiCl (1.9 mL, 15.3 mmol), reflux overnight. After cooling to room temperature, the organic phase was washed successively with dilute hydrochloric acid, saturated sodium bicarbonate, and saturated brine, separated, dried, and concentrated under reduced pressure. The concentrated solution was dropped into a large amount of n-hexane for precipitation, filtered, and vacuum-dried to obtain 2.19 g of a yellow solid with a yield of 89%.
[0062] (2) (Preparation of TMS-PySS-7-ethyl-10-hydroxycamptothecin:
[006...
Embodiment 3
[0069] A reduction-responsive amphiphilic polymer prodrug of 7-ethyl-10-hydroxycamptothecin has a molecular structure as shown below:
[0070]
[0071] where 500
[0072] The preparation method of above-mentioned amphiphilic polymer prodrug is as follows:
[0073] (1) Preparation of TBDPS-7-ethyl-10-hydroxycamptothecin:
[0074] Add 7-ethyl-10-hydroxycamptothecin (2.0 g, 5.1 mmol) into 100 mL of dichloromethane, add triethylamine (3.2 mL, 23 mmol) and TBDPSCl (1.3 mL, 5.1 mmol), and reflux overnight. After cooling to room temperature, the organic phase was washed successively with dilute hydrochloric acid, saturated sodium bicarbonate, and saturated brine, separated, dried, and concentrated under reduced pressure. The concentrated solution was dropped into a large amount of n-hexane for precipitation, filtered, and vacuum-dried to obtain 2.6 g of a yellow solid with a yield of 81%. product 1 H NMR and MS tests showed that TBDPS-7-ethyl-10-hydroxycamptothecin was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com