Telmisartan enteric-coated tablets and preparation method thereof
A technology of telmisartan and enteric-coated tablets is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of cracks in the coating layer, moisture absorption, poor stability and the like, Achieve high dissolution, good application prospects and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
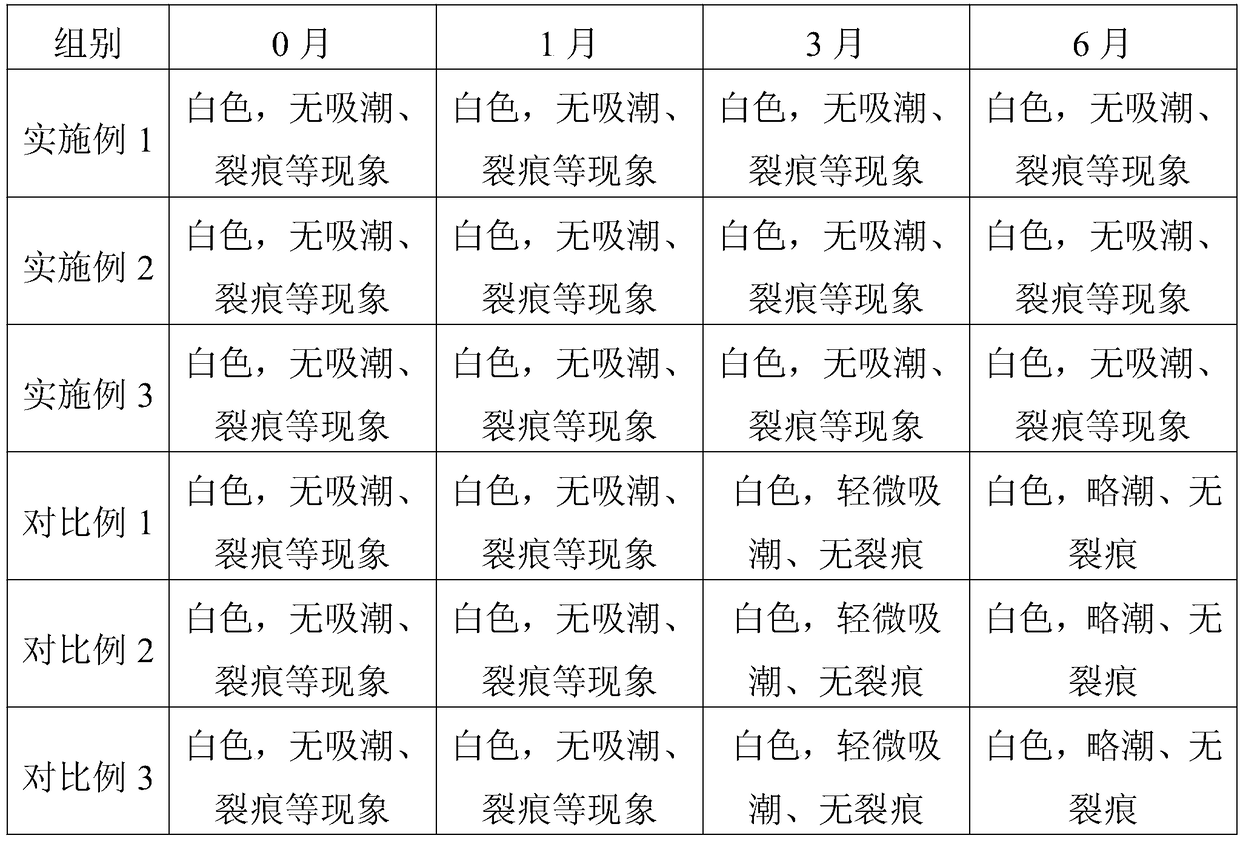
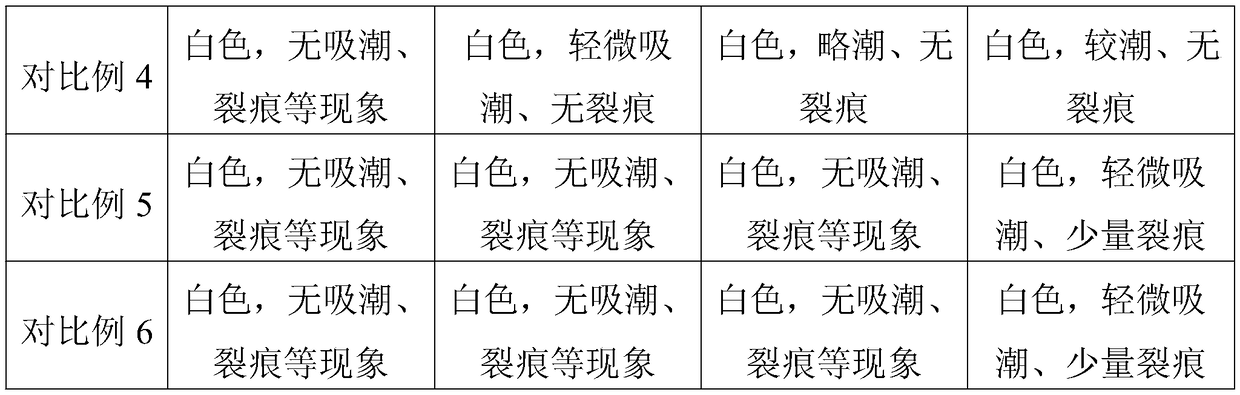
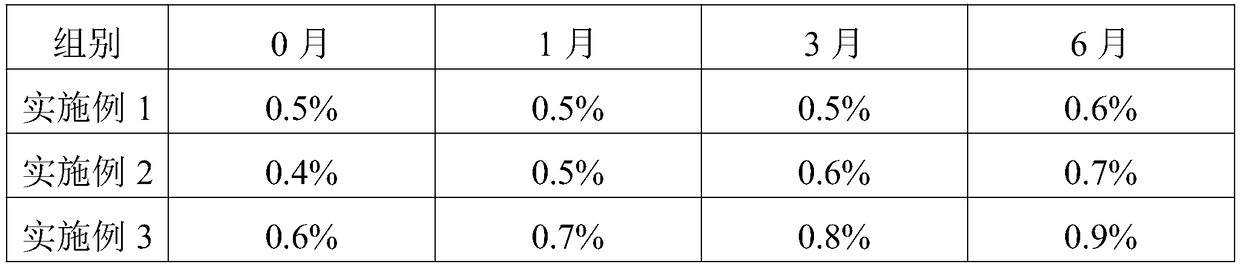
Embodiment 1
[0032] Embodiment 1 A kind of telmisartan enteric-coated tablet
[0033] Consists of a tablet core, an isolation layer and an enteric coating, the tablet core is made of the following raw materials and their parts by weight: 20 parts of telmisartan, 8 parts of polyarginine with a molecular weight of 2000D, and 20 parts of mannitol , 60 parts of microcrystalline cellulose, 4 parts of povidone and 1 part of magnesium stearate.
[0034] Preparation steps:
[0035] 1, preparation of tablet core: get corresponding amount of telmisartan, polyarginine and mannitol and dissolve in the purified water that weight volume ratio is 1:10, stir 10min at 55 ℃, spray dry to get fine powder, add corresponding amount of Microcrystalline cellulose, povidone and magnesium stearate are uniformly mixed, placed in a tablet press for tableting, to obtain tablet cores;
[0036] II. Prepare the isolation layer: take the immediate-release coating powder and disperse it evenly in purified water to make...
Embodiment 2
[0038] Embodiment 2 A kind of telmisartan enteric-coated tablet
[0039] Consists of a tablet core, an isolation layer and an enteric coating, the tablet core is made of the following raw materials and their parts by weight: 30 parts of telmisartan, 12 parts of polyarginine with a molecular weight of 1500D, and 15 parts of mannitol , 80 parts of microcrystalline cellulose, 5 parts of povidone and 2 parts of magnesium stearate.
[0040] Refer to Example 1 for the remaining parameters and preparation steps.
Embodiment 3
[0041] Embodiment 3 A kind of telmisartan enteric-coated tablet
[0042]Consists of a tablet core, an isolation layer and an enteric coating, the tablet core is made of the following raw materials and their parts by weight: 40 parts of telmisartan, 16 parts of polyarginine with a molecular weight of 2000D, and 20 parts of mannitol , 60 parts of microcrystalline cellulose, 6 parts of povidone and 1 part of magnesium stearate.
[0043] Refer to Example 1 for the remaining parameters and preparation steps.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com