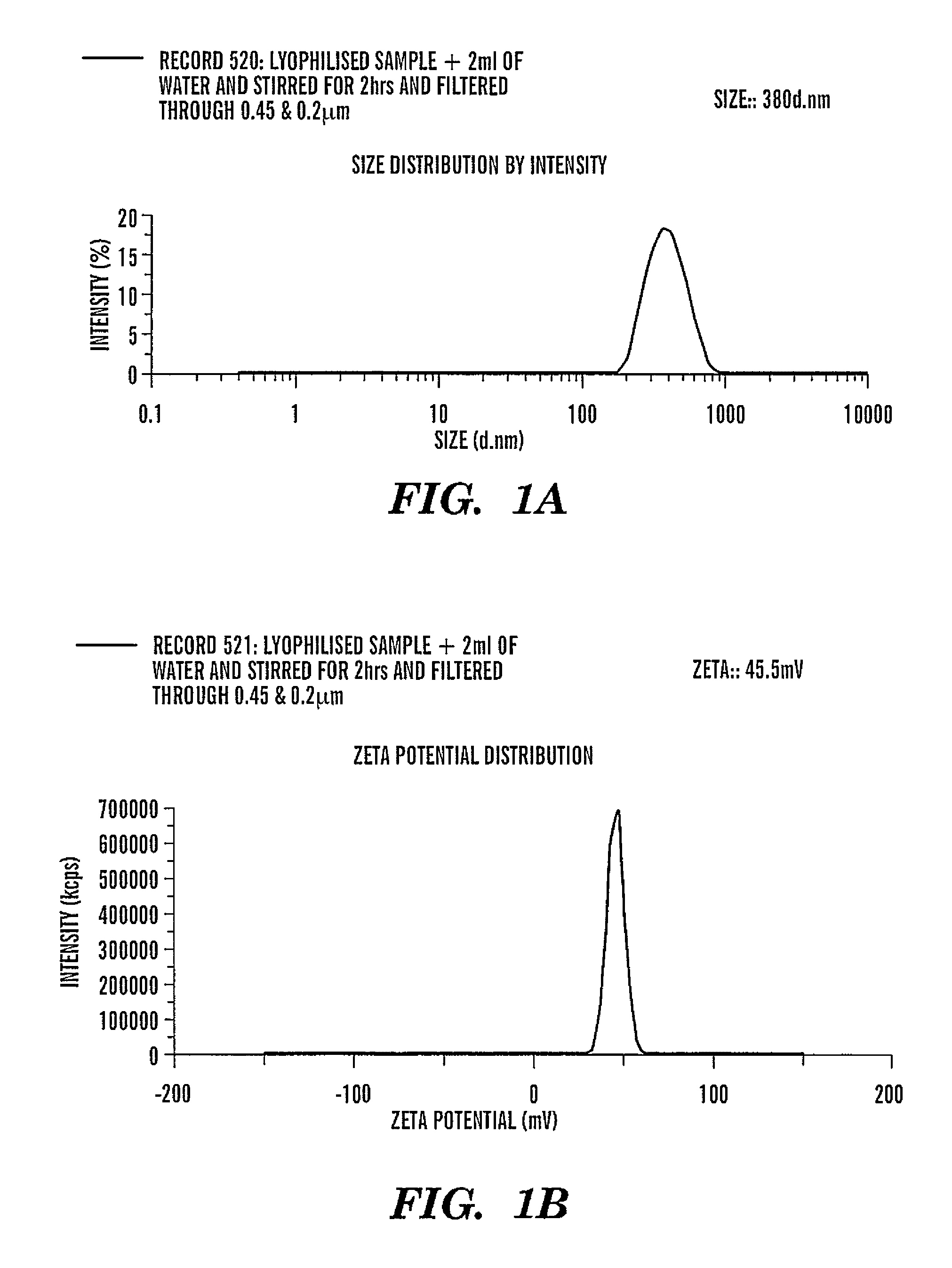
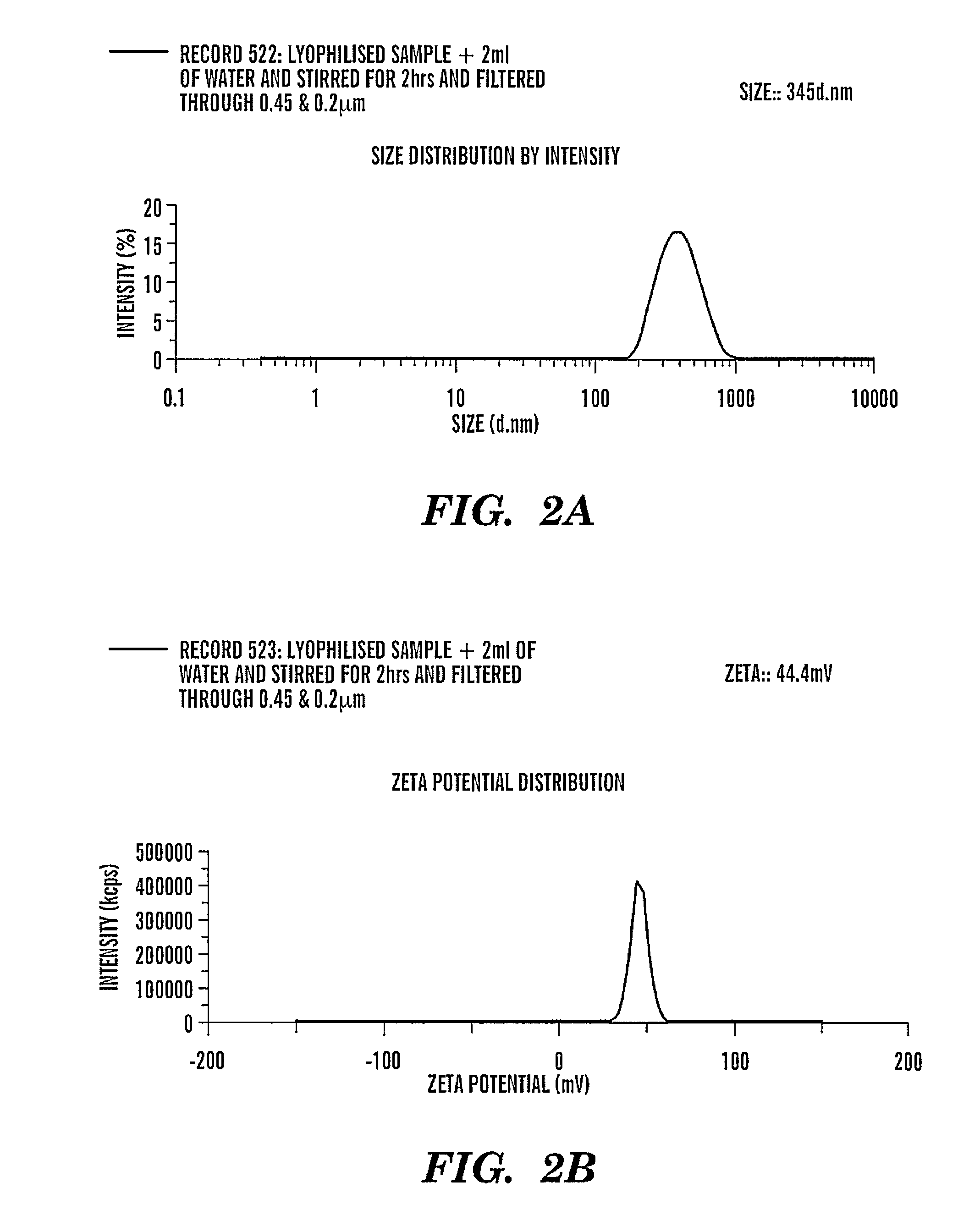
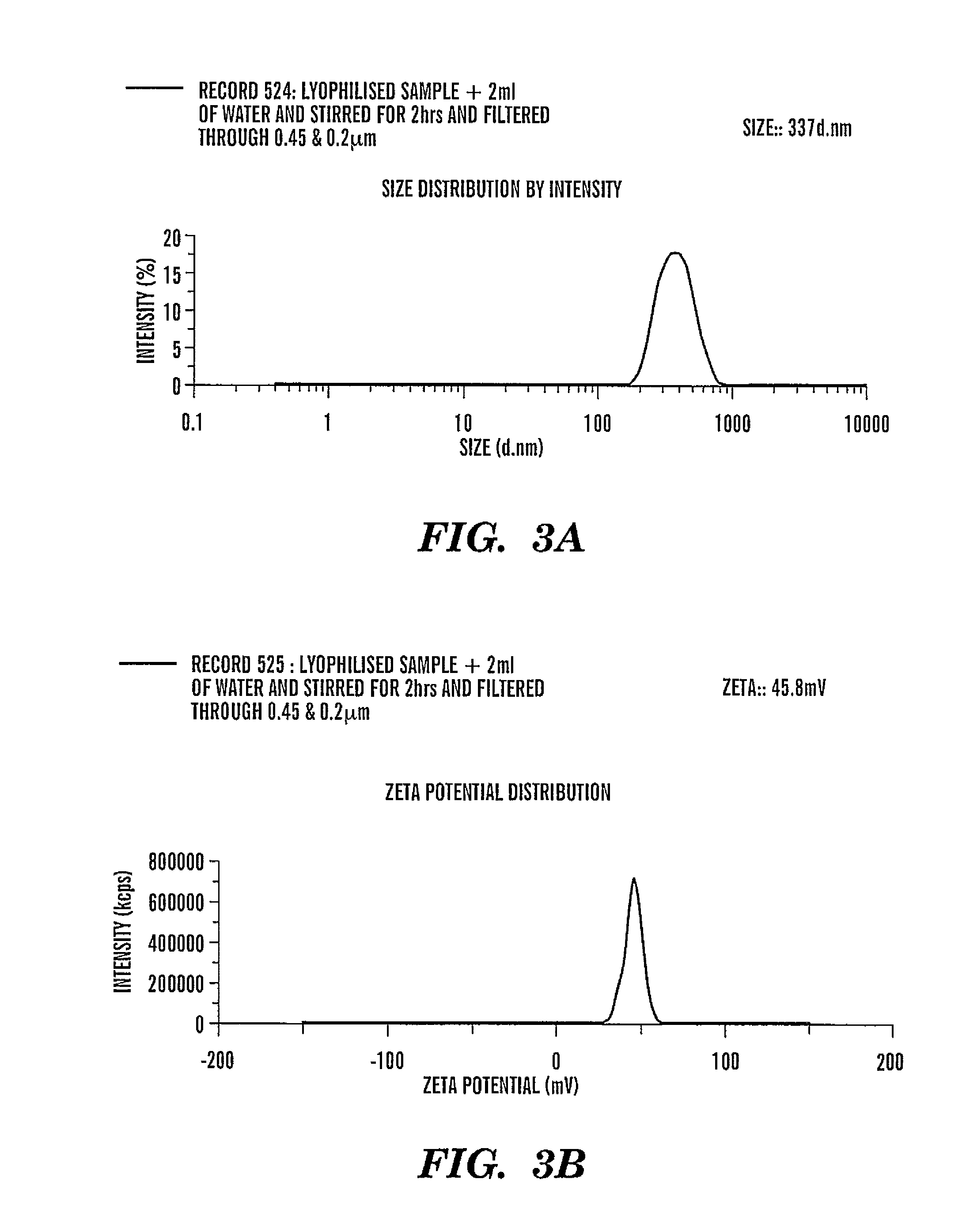
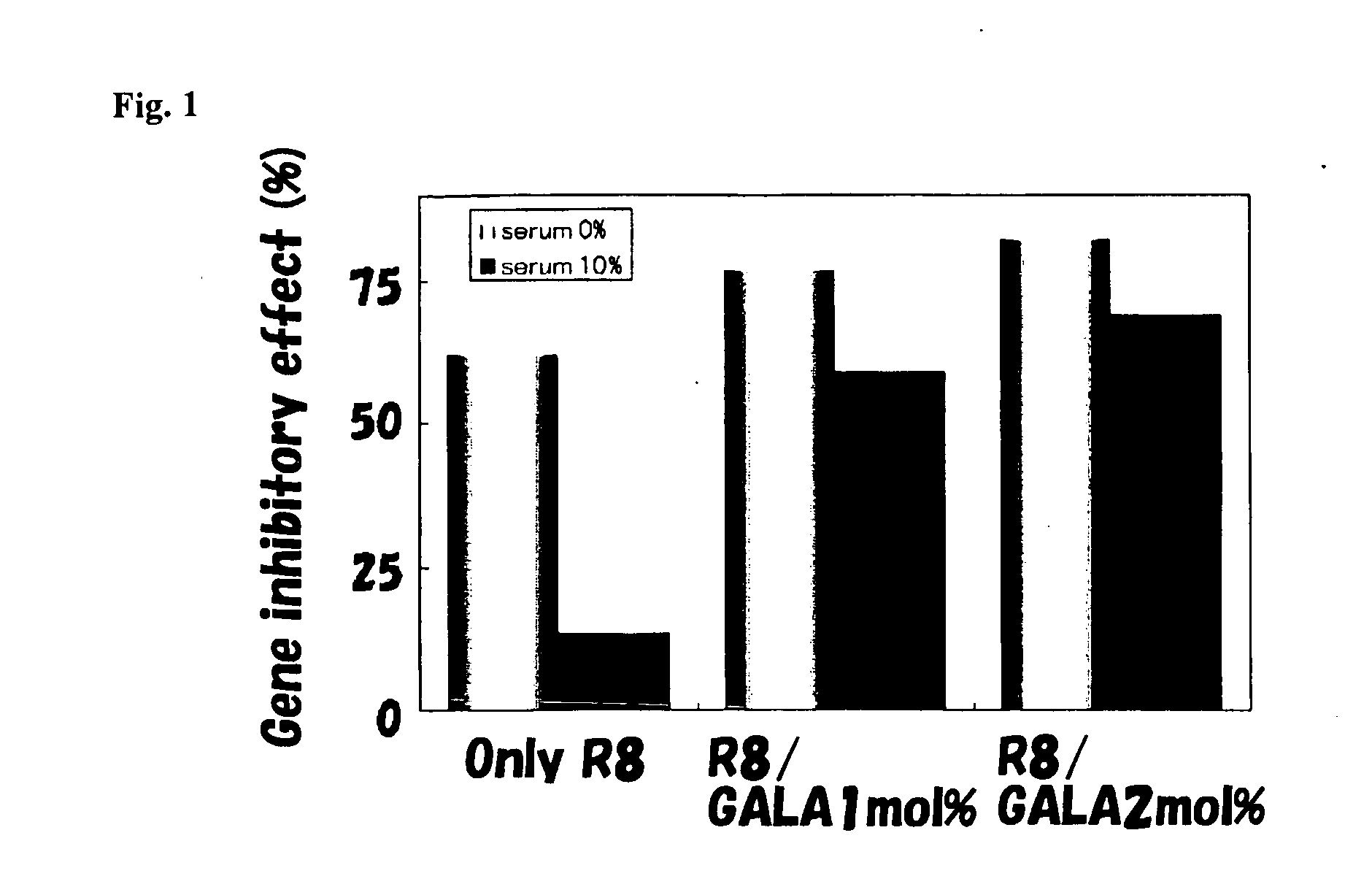
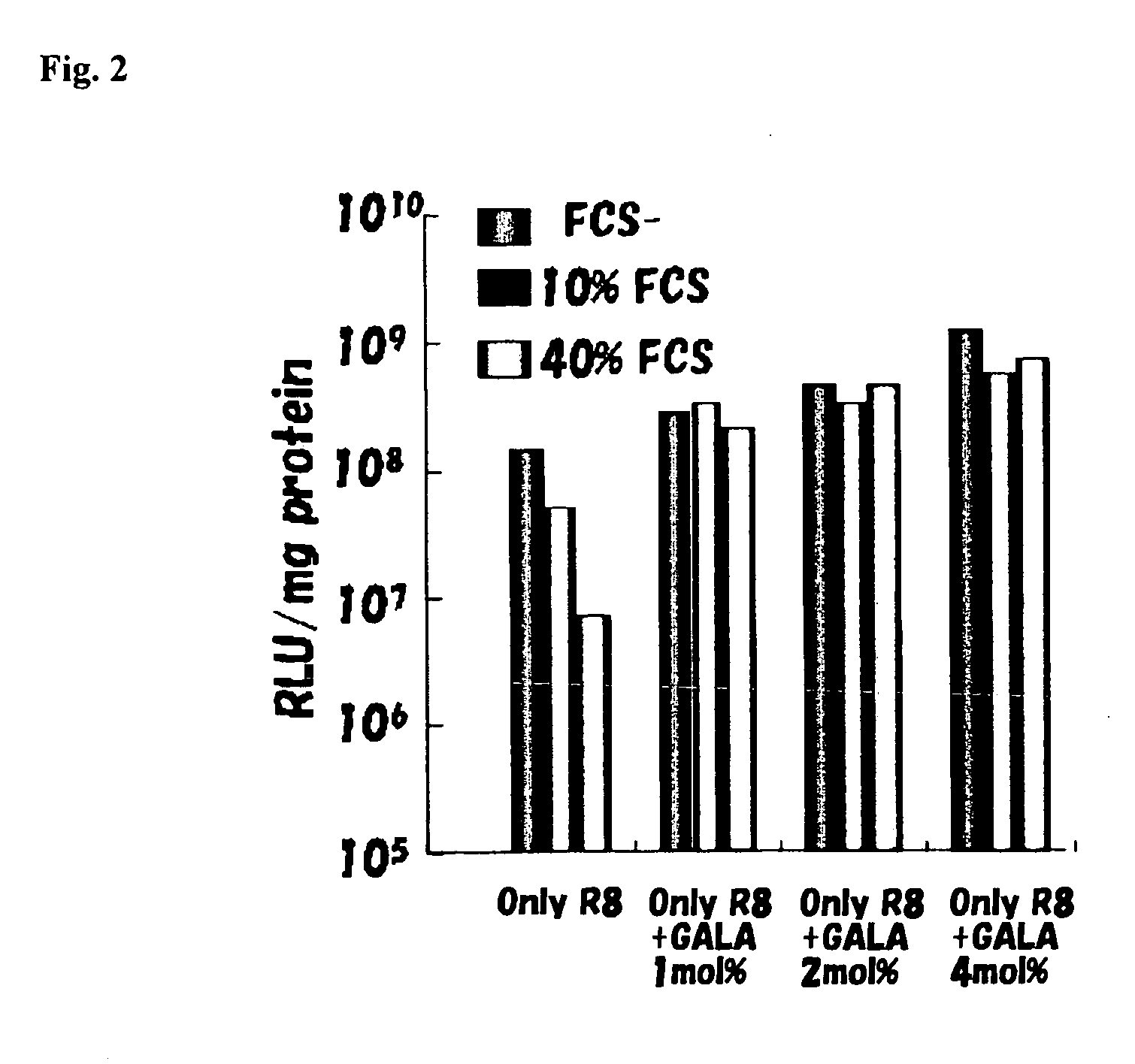
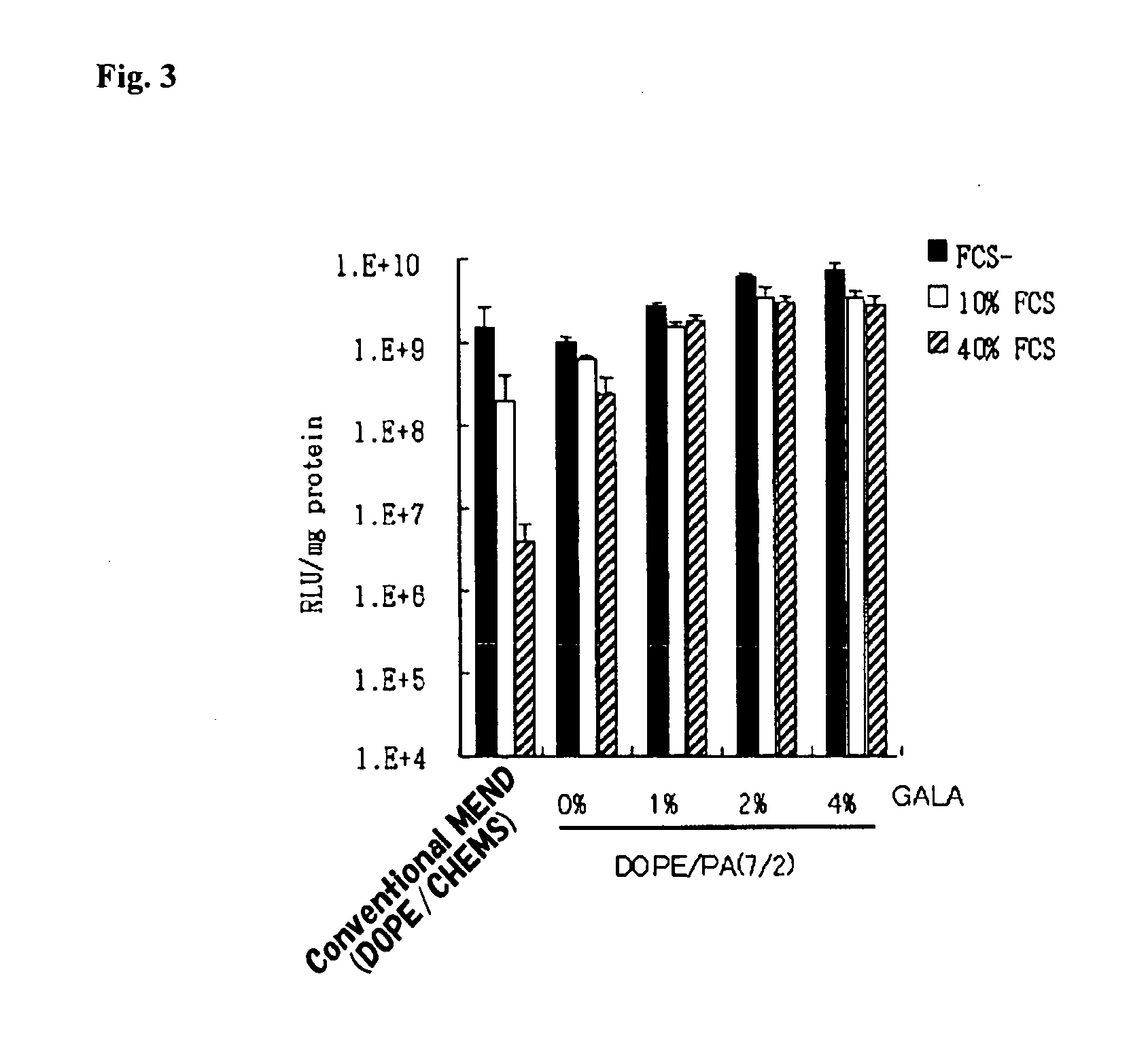
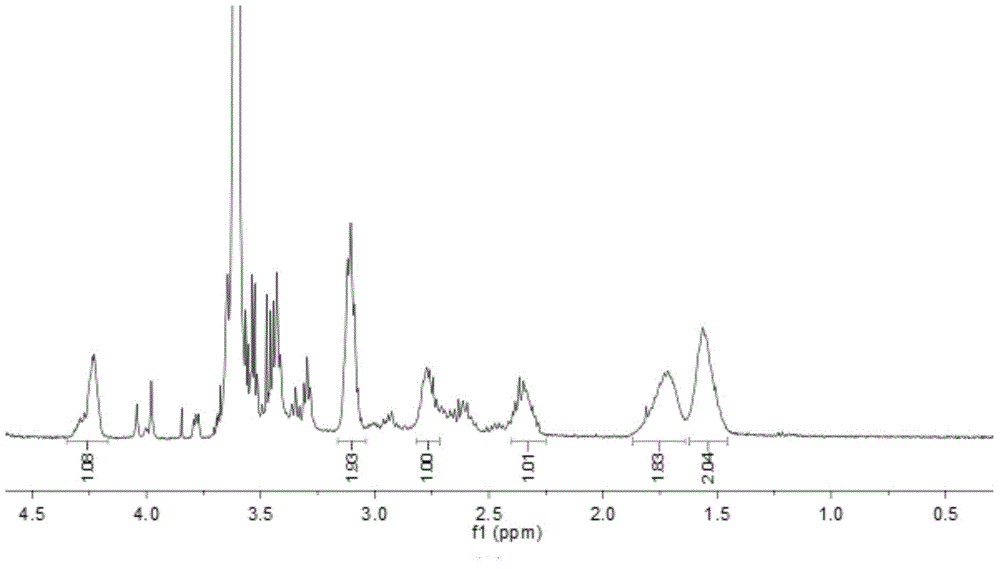
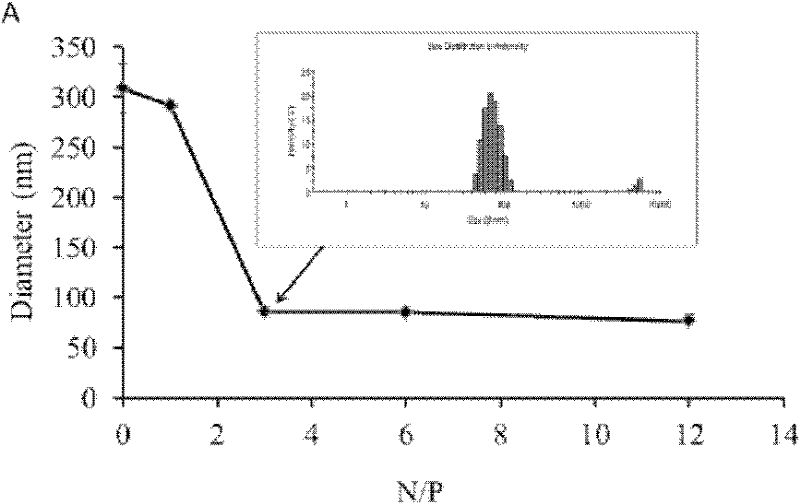
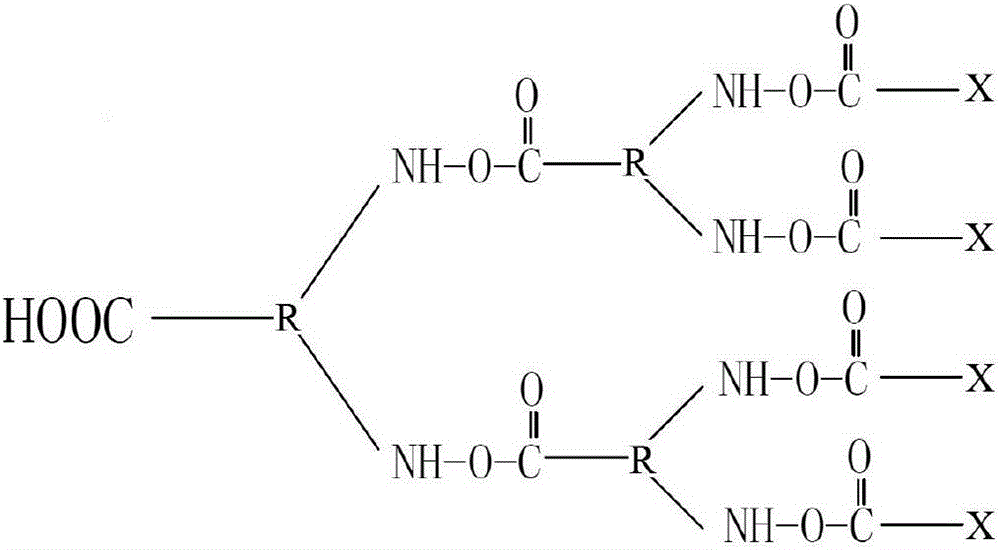
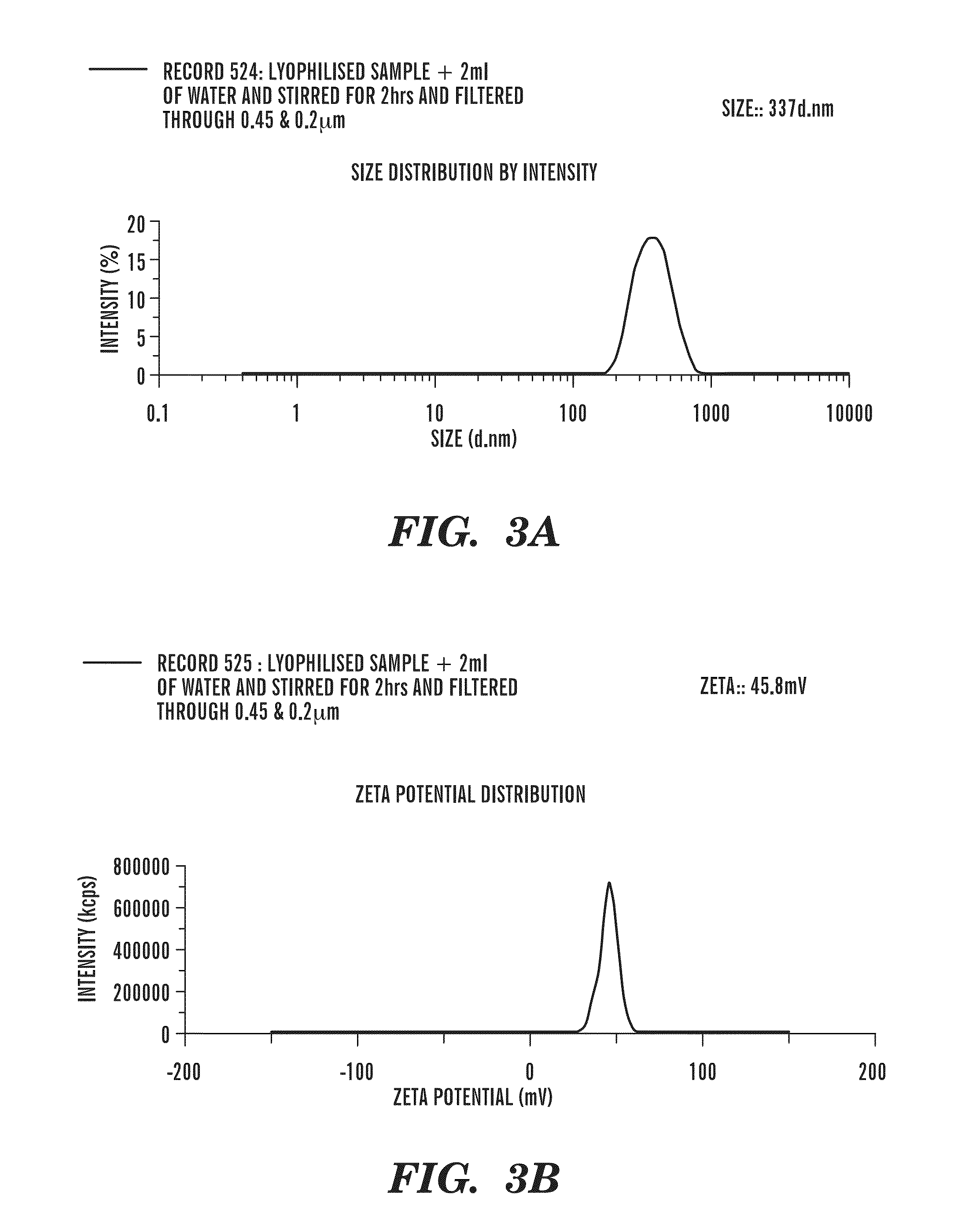
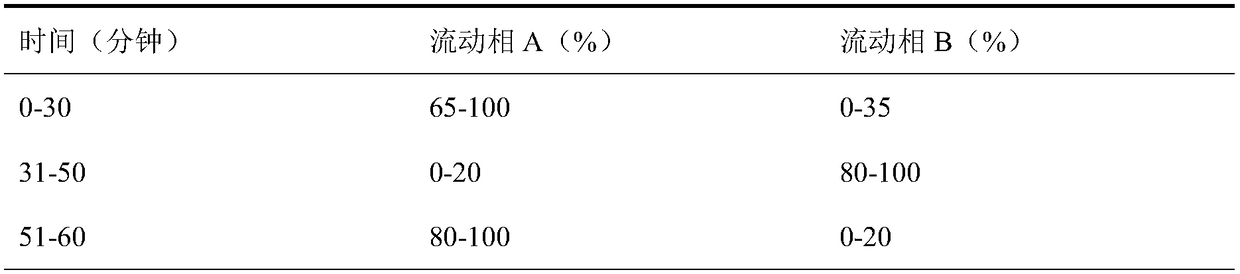
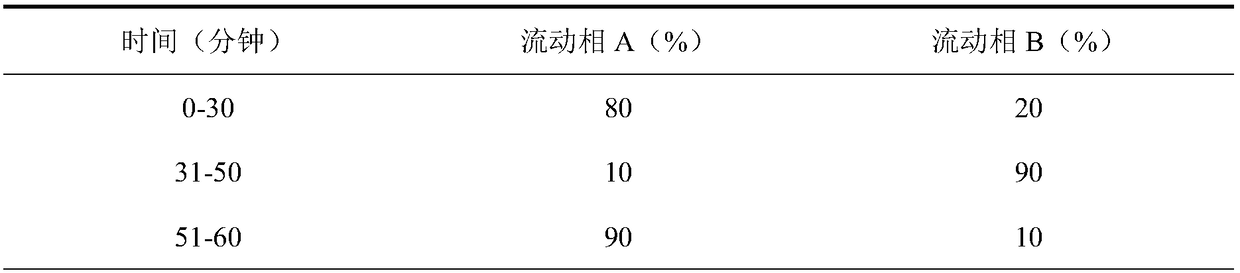
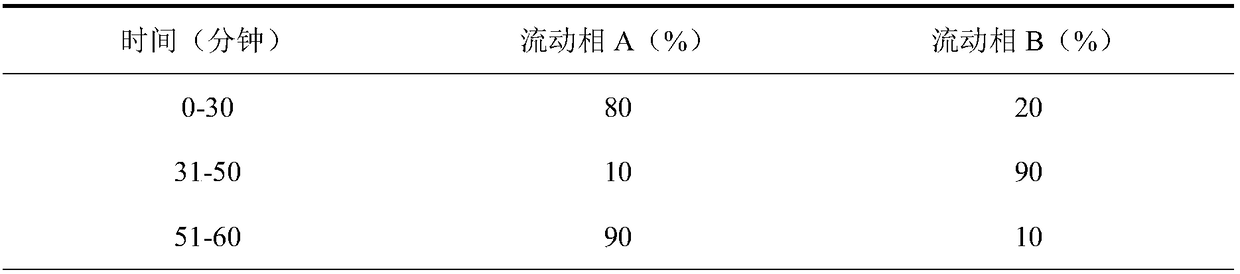
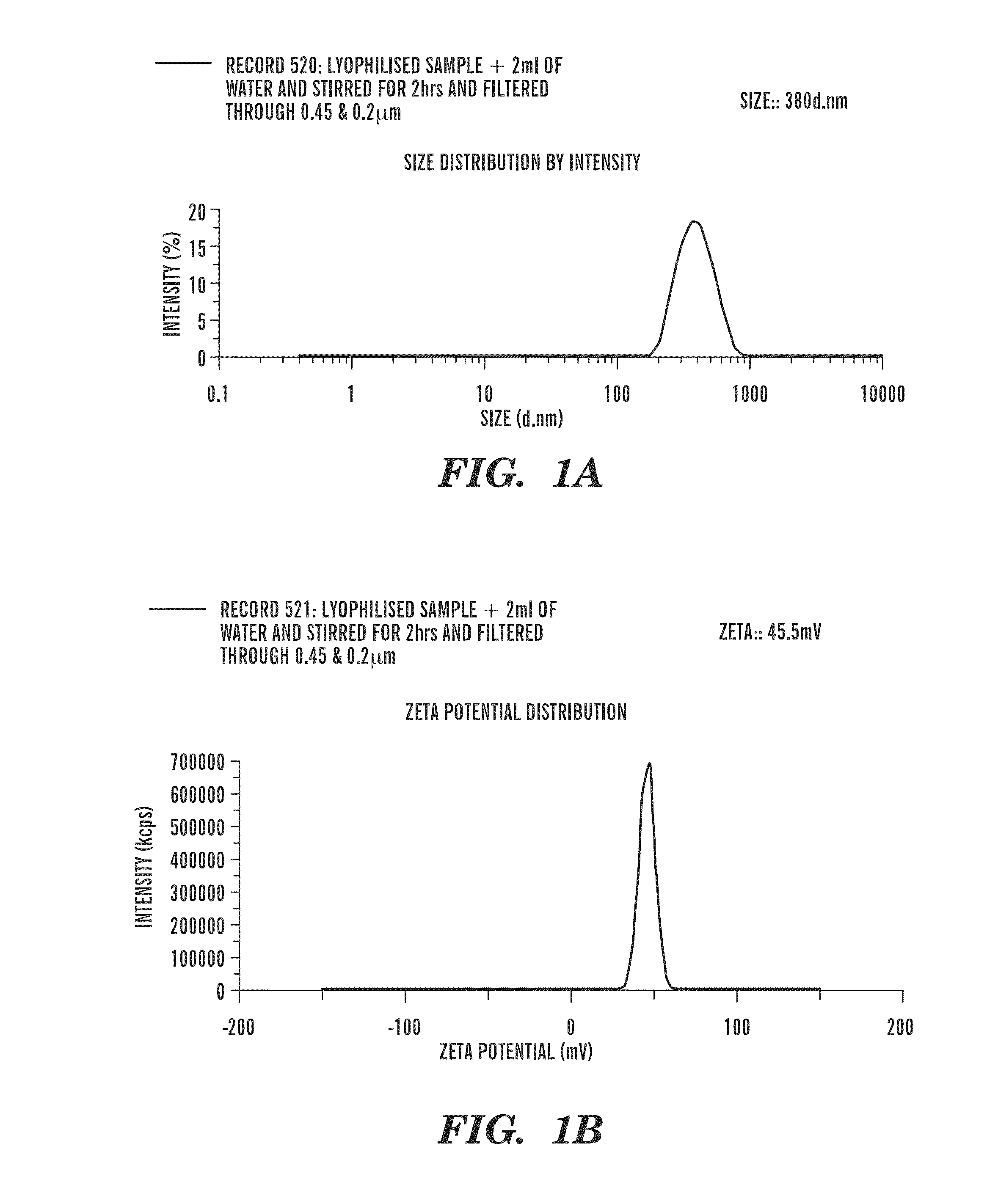
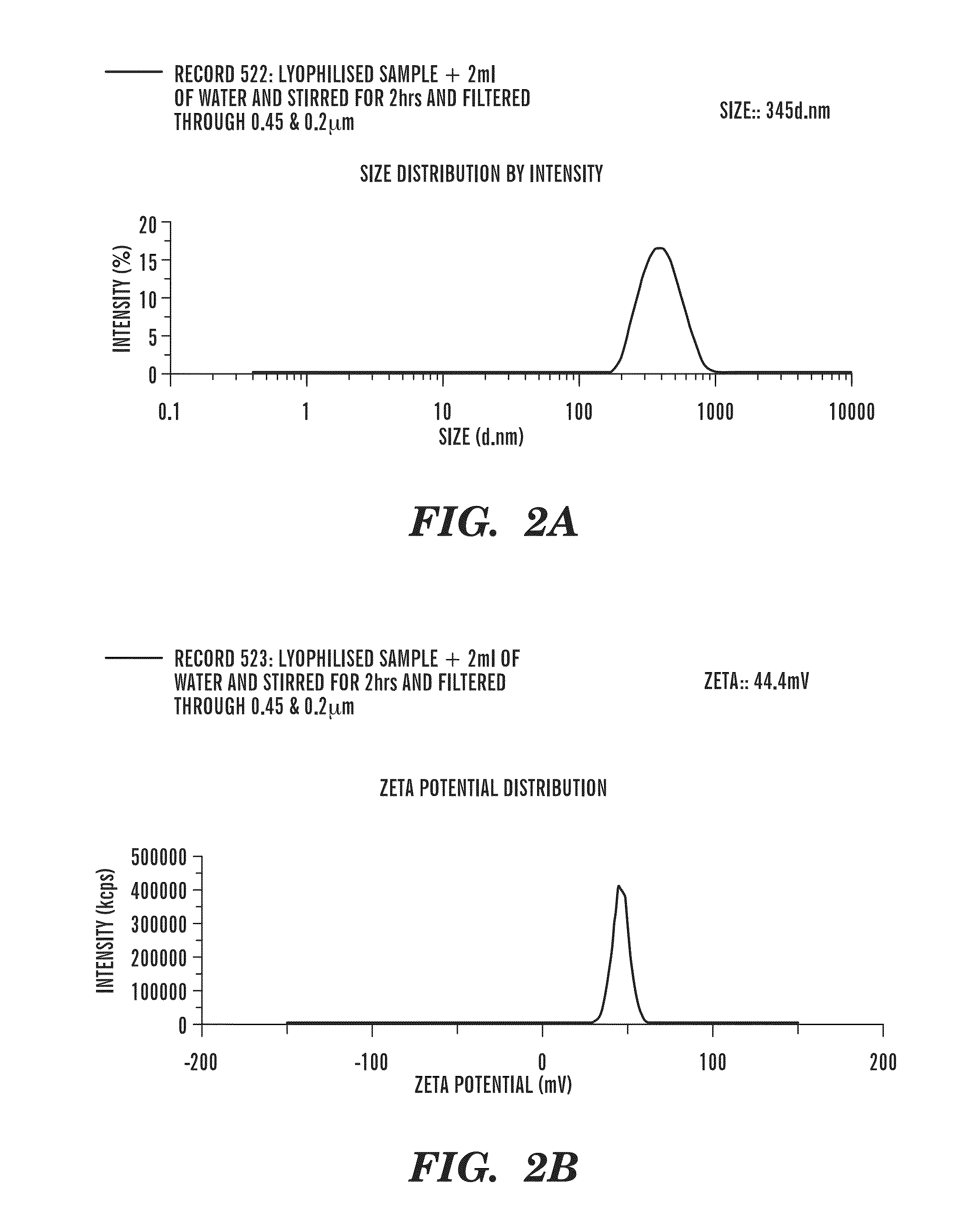
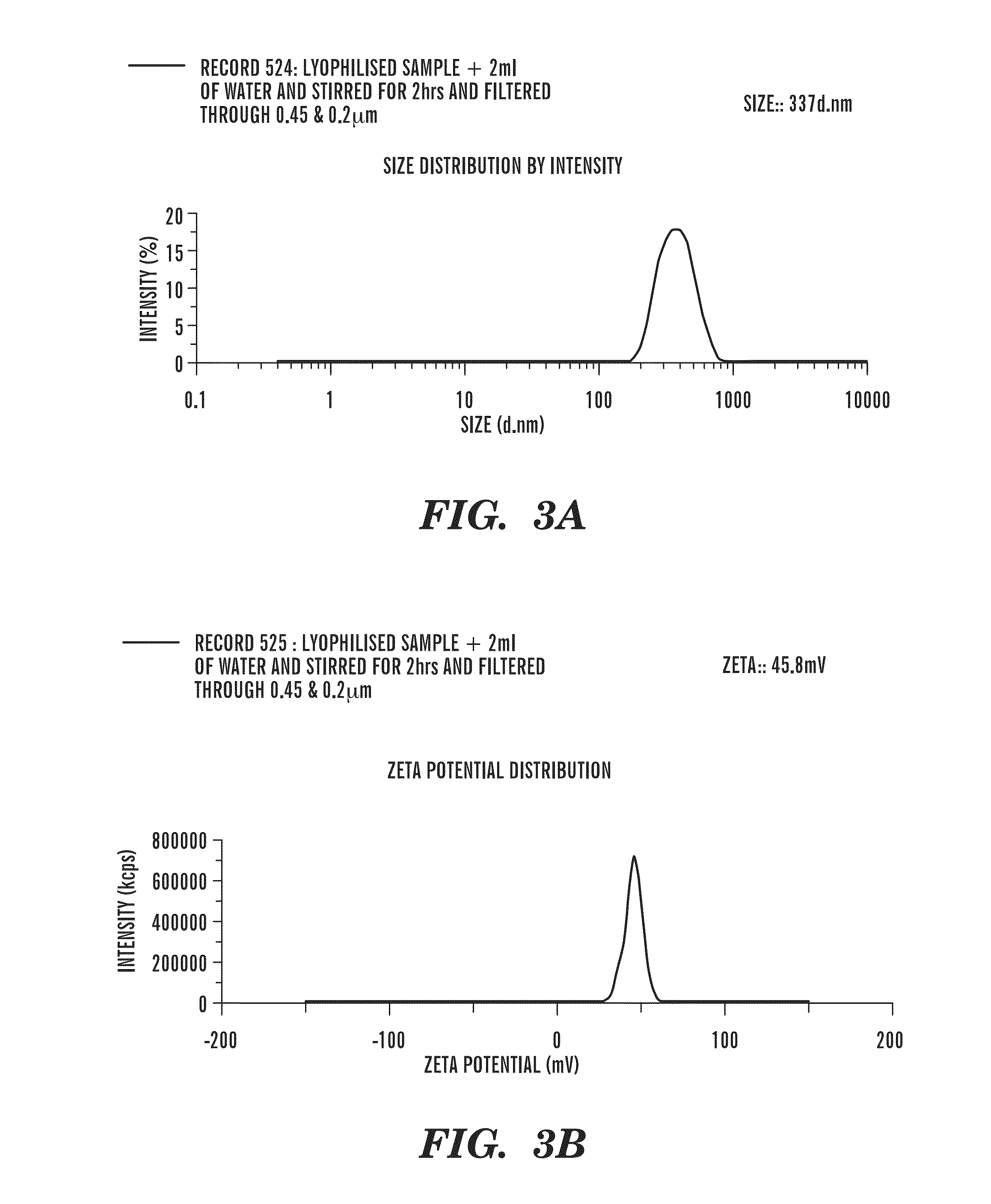
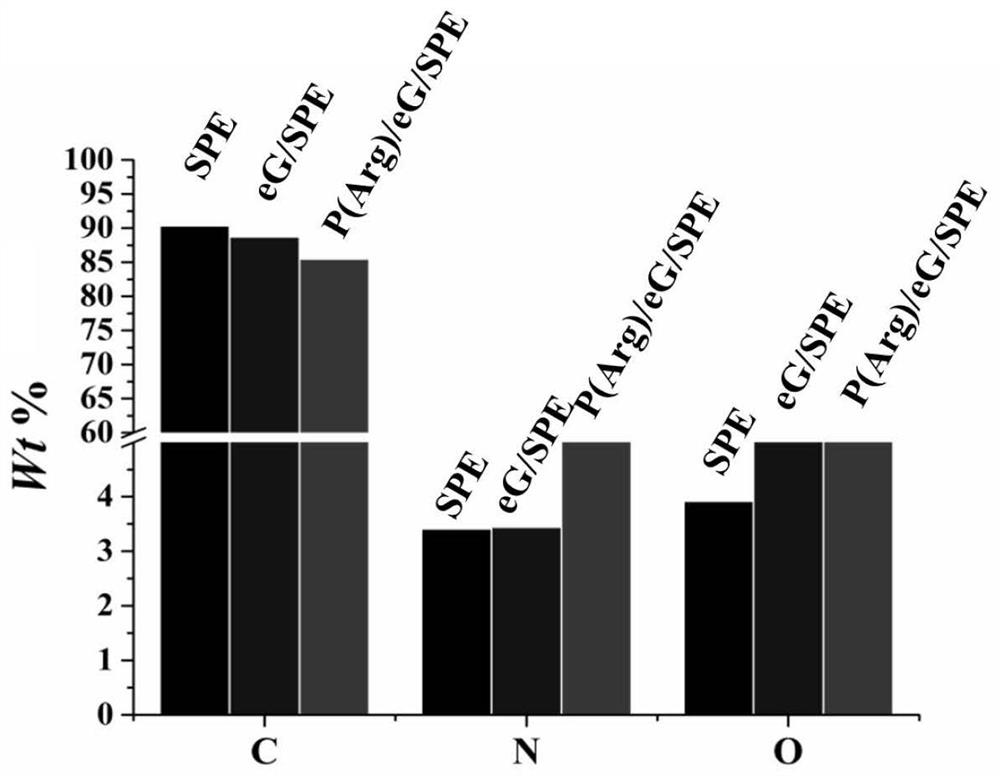
Patents
Literature
63 results about "Poly l arginine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
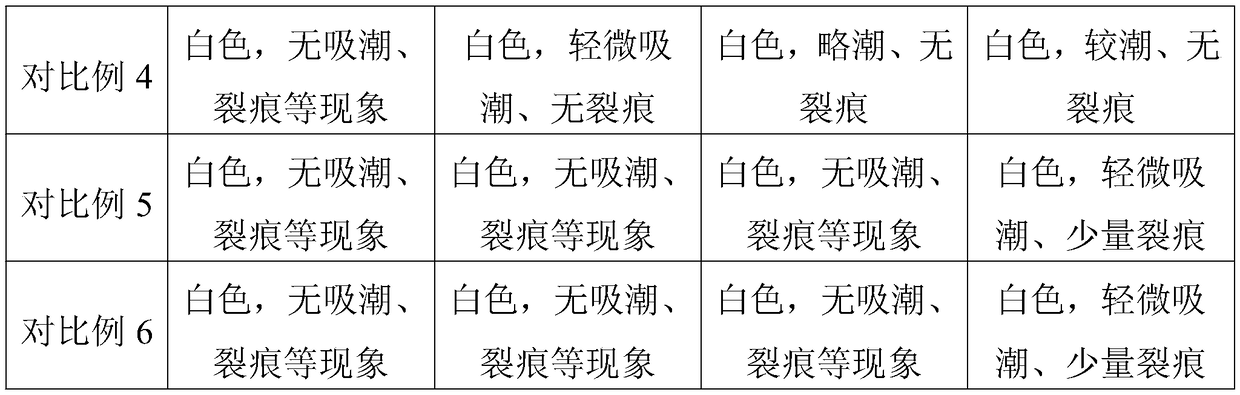
Therapeutic composition and a method of coating implantable medical devices
A therapeutic composition is provided including a polysaccharide or a cationic peptide dissolved in an organic substance. The polysaccharide can be heparin or a derivative of heparin. The cationic peptide can be L-arginine, oligo-L-arginine or poly-L-arginine. The organic substance can be formamide. A method of coating an implantable medical device is also provided, comprising applying the therapeutic composition to the device and allowing the organic substance to evaporate. The device can be a stent.
Owner:ABBOTT CARDIOVASCULAR
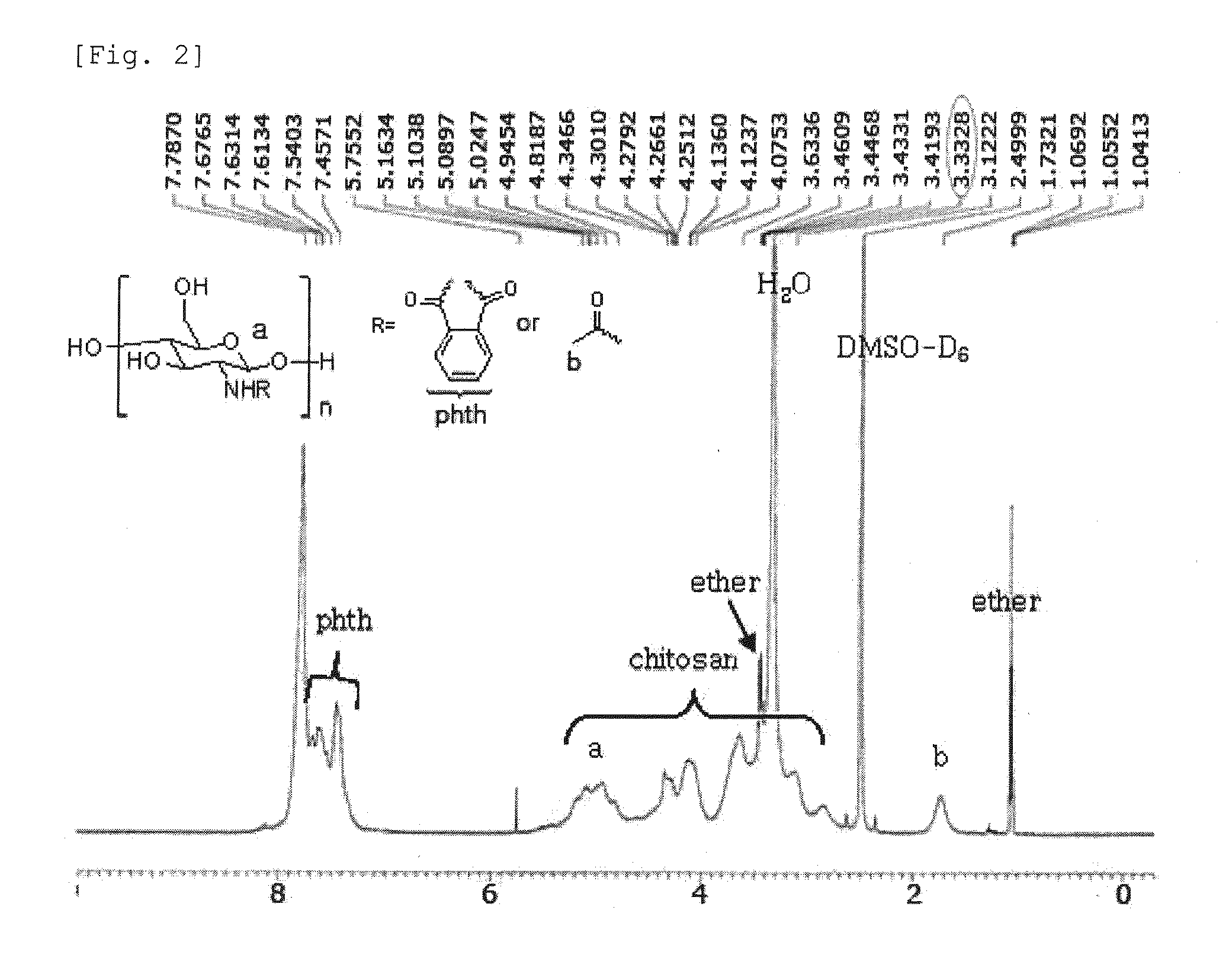
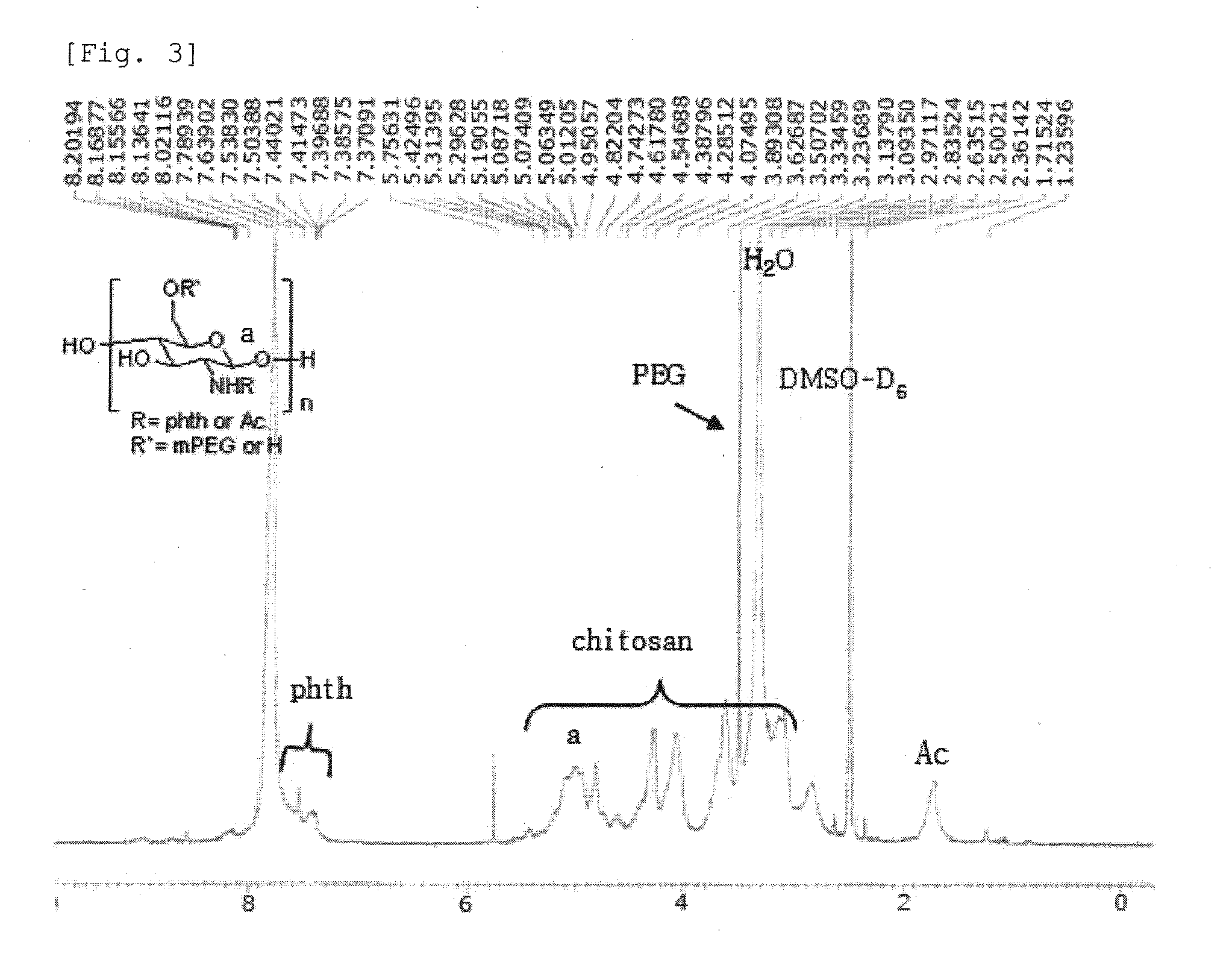
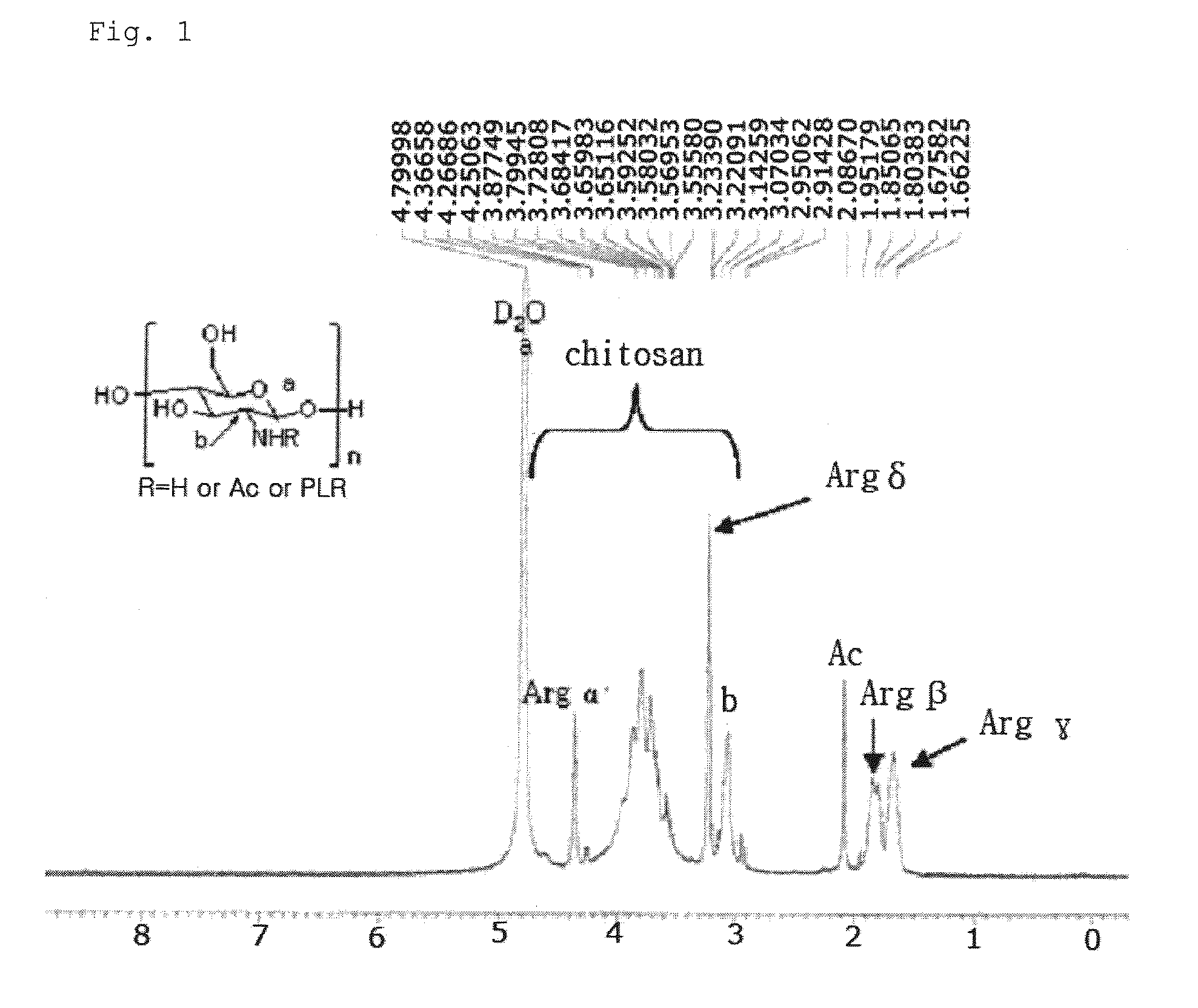
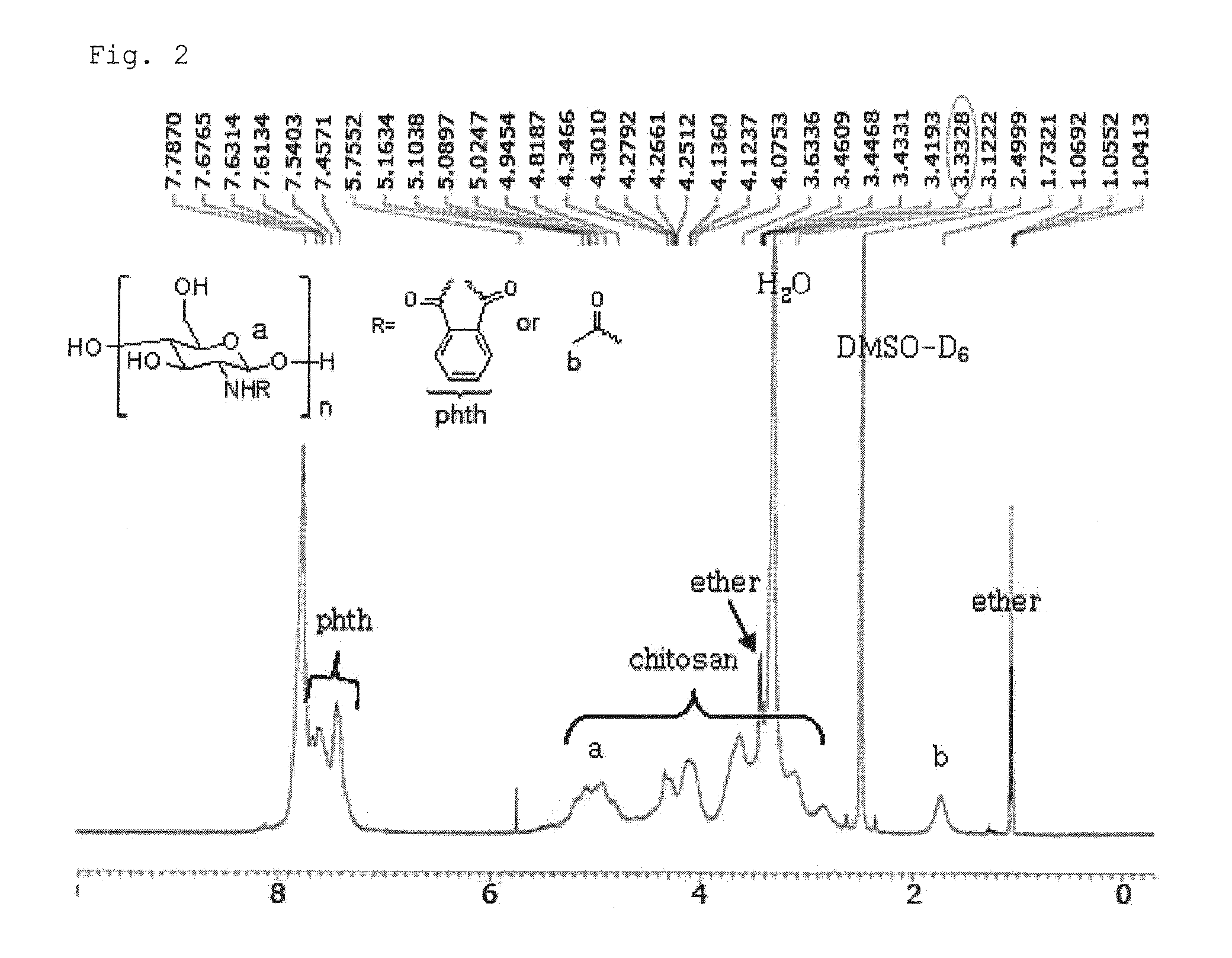
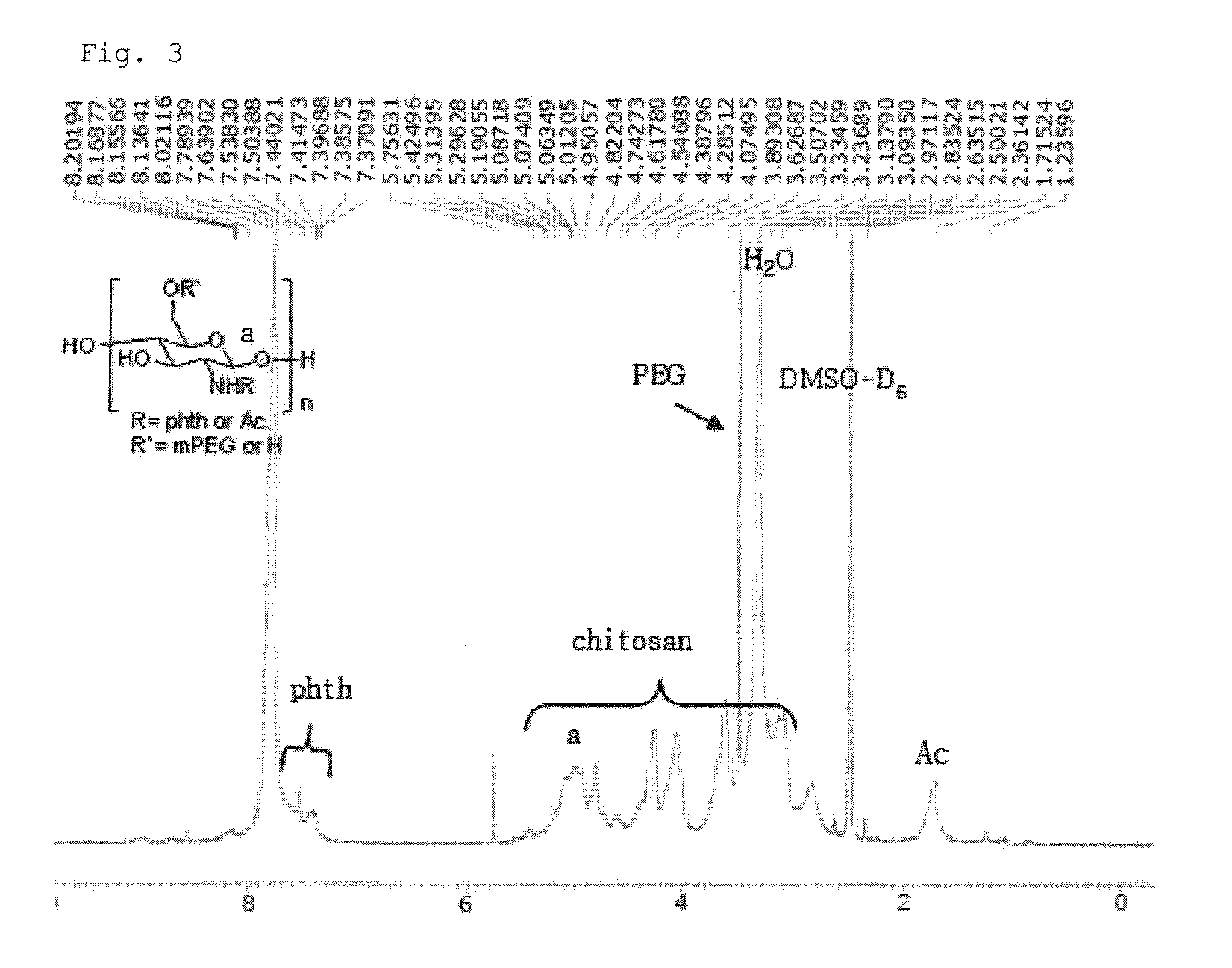
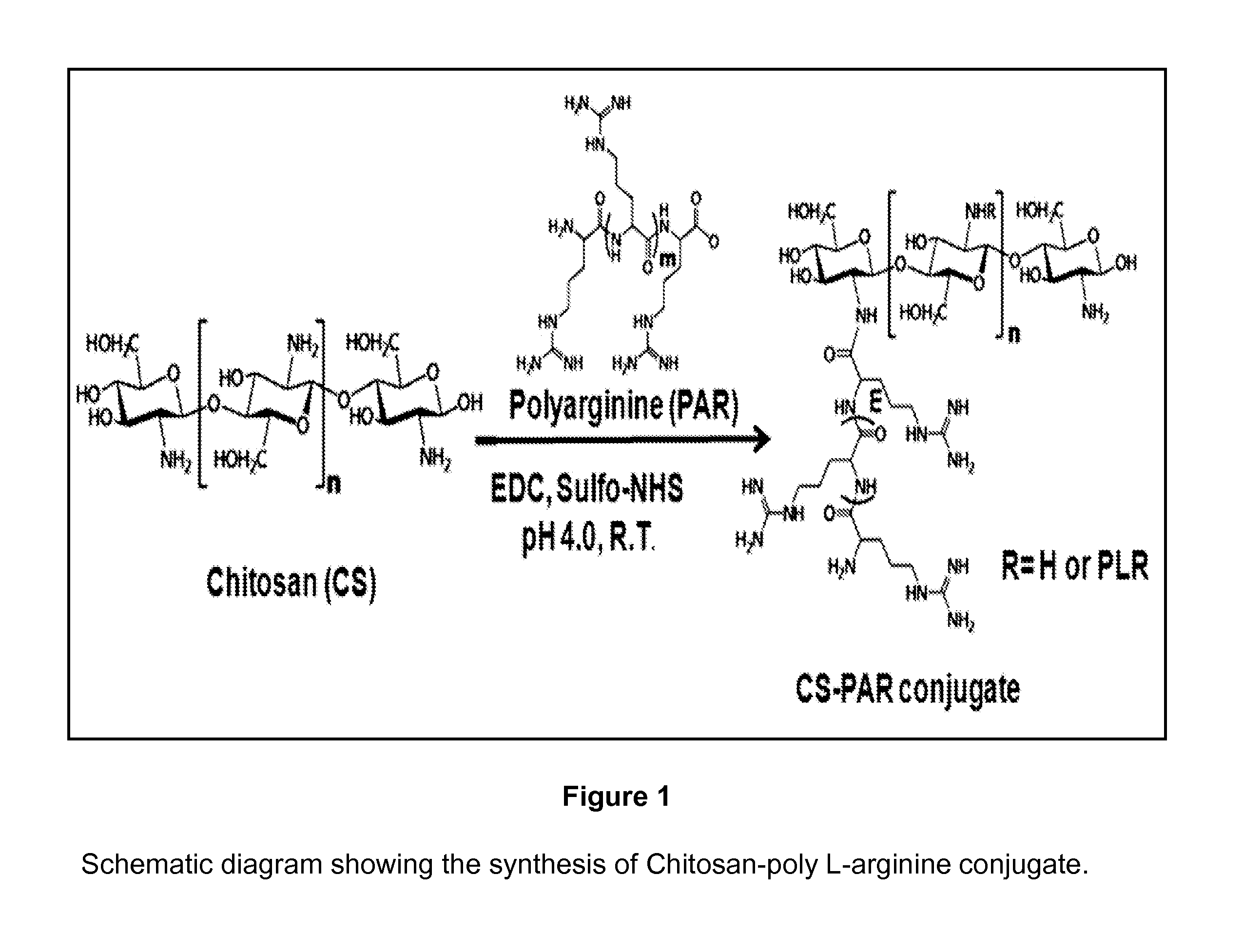
Chitosan Based Polymer Conjugate and a Method for Producing the Same
InactiveUS20100113559A1Improve in vivo transfer efficiencyLow cytotoxicityPeptide/protein ingredientsGenetic material ingredientsPolyethylene glycolCytotoxicity
The present invention relates to a conjugate of chitosan and polyamine polymer that is useful for transferring a desired gene medicine into cells, and a method for preparing the same. In particular, the present invention relates to a double conjugate that is prepared by 1 in king poly-L-arginine to low molecular weight chitosan or triple conjugate that is prepared by additionally linking polyethylene glycol (PEG) to the double conjugate, and a method for preparing the same. The chitosan based cationic polymer conjugate of the present invention forms a complex with negatively charged gene medicine such as plasmid DNA and small interfering RNA to efficiently transfer the desired gene medicine into cells with low cytotoxicity. Accordingly, the conjugate can be used as an effective delivery system for in vivo administration of gene medicine.
Owner:ENGENE INC
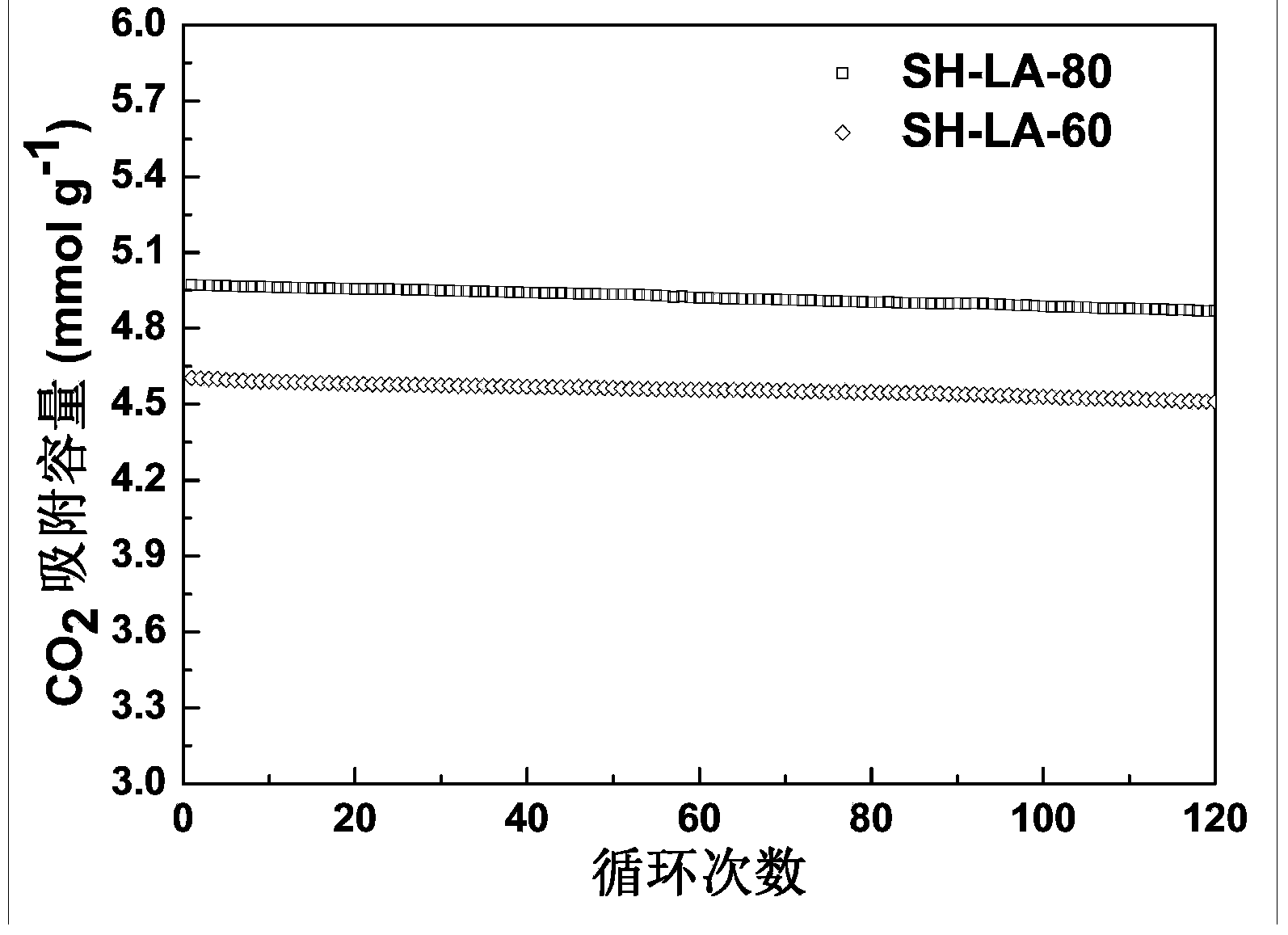
Carbon dioxide adsorbent and preparation method thereof
InactiveCN104258828ASolve the problem of poor long-term stabilityHigh molecular weightProductsOther chemical processesIn situ polymerizationSorbent
The invention discloses a carbon dioxide solid amine adsorbent and a preparation method thereof. The adsorbent comprises a silicon dioxide hollow ball carrier with a large hole size (50nm-550nm) and linear poly-L-arginine (PLA) which is chemically bonded on the inner and outer surfaces of a hollow ball by an in situ polymerization manner. The specific preparation method comprises the following steps: (1) synthesizing the silicon dioxide hollow ball carrier by using tetraethoxysilane and polystyrene emulsion (PS); (2) carrying out amination on the surface of the hollow ball carrier with 3-aminopropyltrimethoxysilane; and (3) polymerizing PLA on the inner and outer surfaces of the hollow ball in situ by adopting the in situ polymerization manner. The adsorbent has the remarkable advantages of high adsorption-desorption speed, large adsorption capacity, good long-period stability and the like; the problems of corrosion and high energy consumption of a liquid ammonia adsorbent in current industry are solved; and the problems of the solid amine adsorbent that the long-period adsorptivity is poor and the adsorption capacity is low are also solved.
Owner:QINGDAO UNIV OF SCI & TECH
Double-layer microencapsulation prebiotic and probiotic composition and preparation method thereof
PendingCN109674061AReduced activityGuaranteed stabilityMilk preparationFood shapingD-GlucoseGlucose polymers
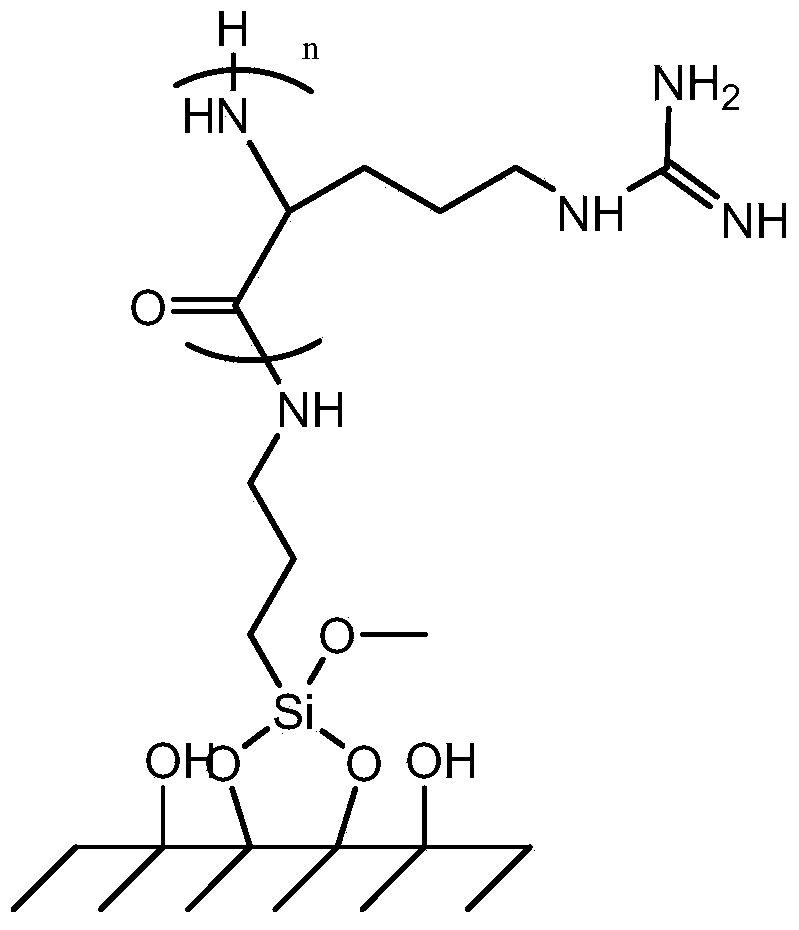
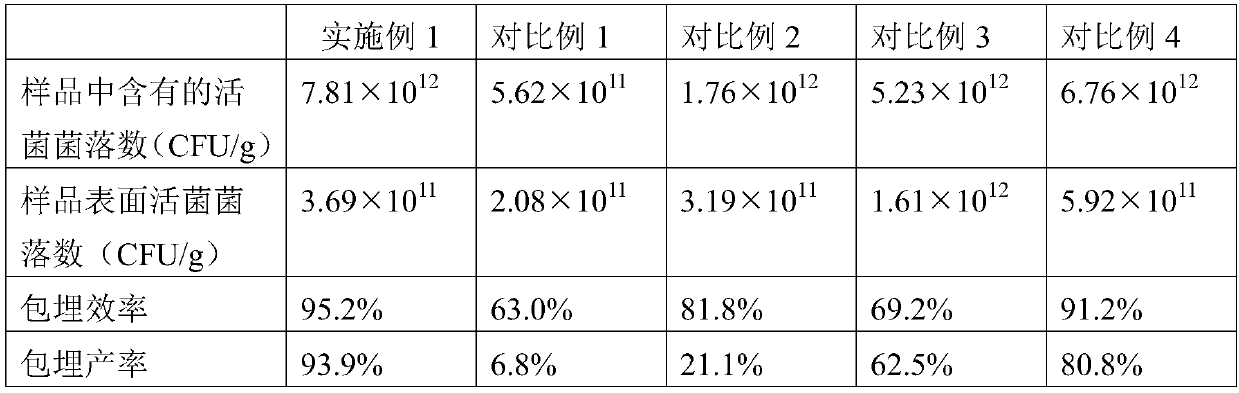
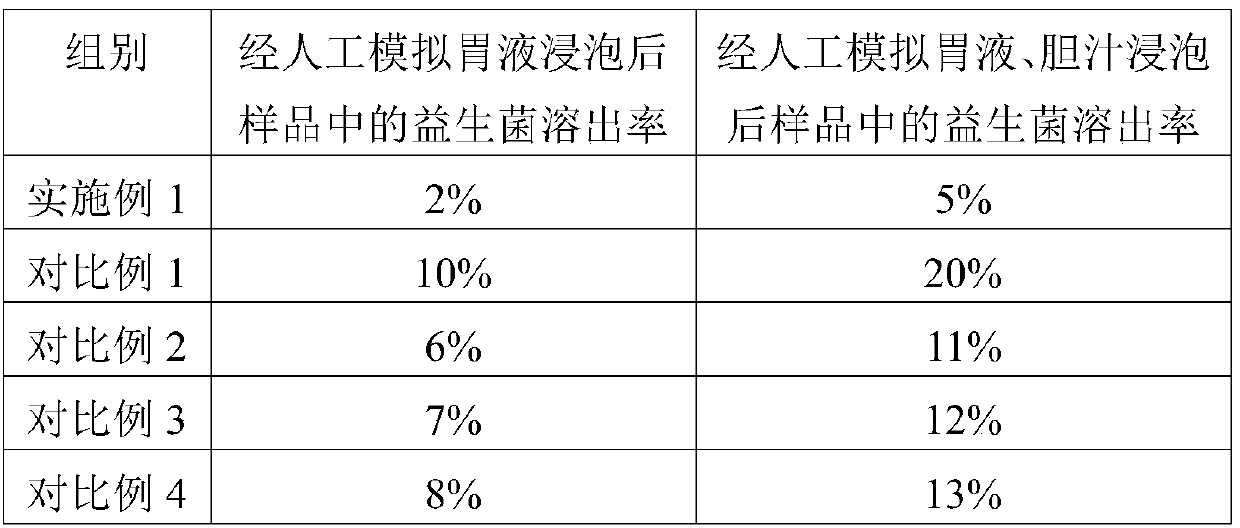
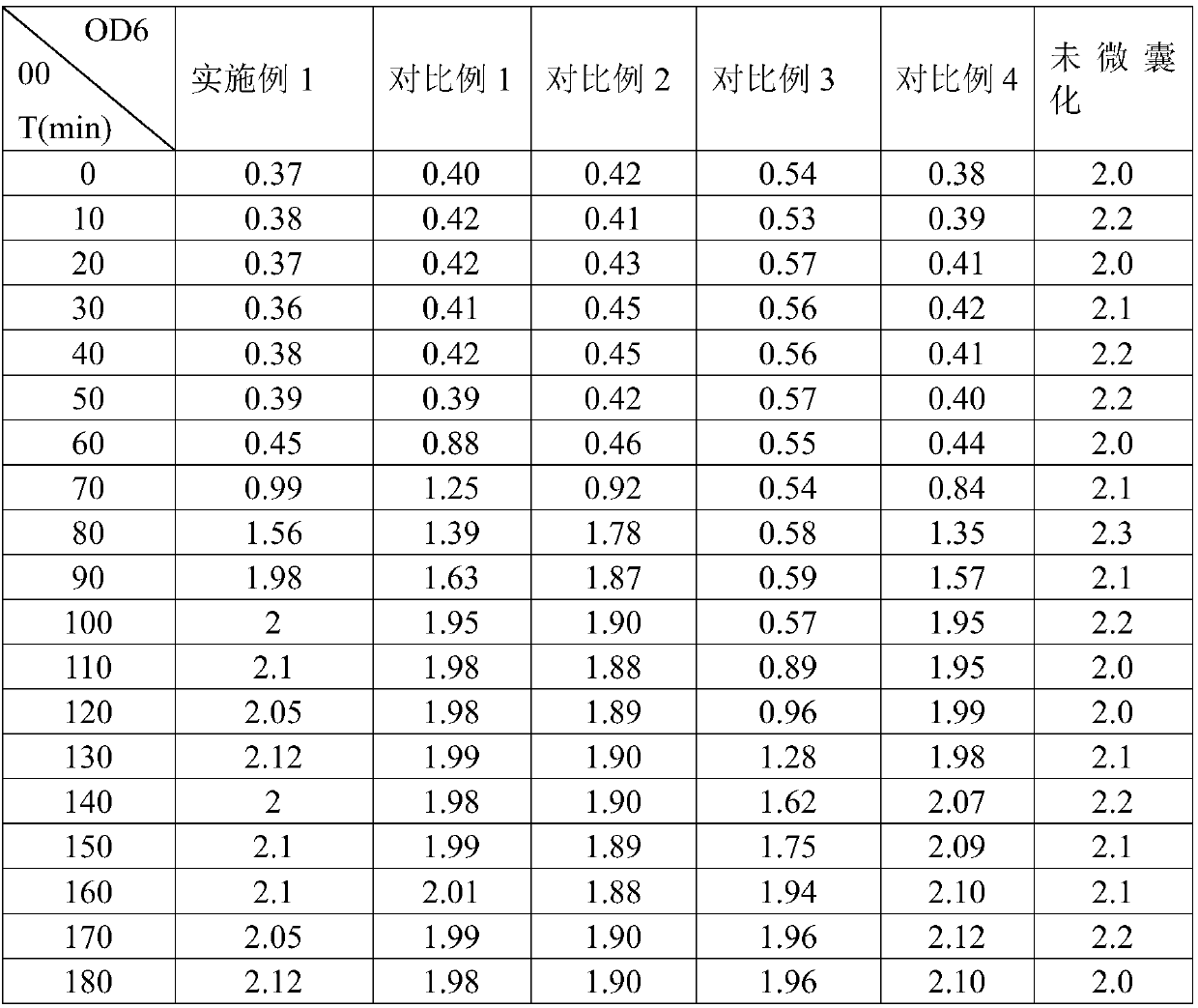
The invention provides a double-layer microencapsulation prebiotic and probiotic composition and a preparation method thereof. A microencapsulation protection technology is used; natural polymer sodium alginate, glucose and prebiotics are used as outer wall materials; poly-L-arginine and prebiotics are used as inner wall materials; probiotics and dried skim milk are used as core materials. The specific double-layer microencapsulation structure provides dual protection for unstable probiotics; the influence of external environment and adverse factors (such as light, temperature, humidity, pH and bile) is avoided; the reduction of probiotic activity in the use and storage processes is effectively prevented; the stability, the activity and the effective fix planting rate of the composition are maintained to the greatest degree; the embedding efficiency is high; the embedding effect is good. The double-layer microencapsulation composition has the advantages that the directional controlledrelease effect can be effectively realized; the intestinal flora balance can be effectively reduced; the body immunity is improved.
Owner:山东探克生物科技股份有限公司
Tumor targeting metal complex, synthetic method and application
ActiveCN106866743AImprove utilizationSmall toxicityRuthenium organic compoundsOrganic active ingredientsAbnormal tissue growthApoptosis
The invention discloses a tumor targeting metal complex, a synthetic method and application. The synthetic method comprises the steps of connecting a ligand with a substituent group and a targeting molecule, complexing the ligand and metal ions, and synthesizing to obtain the tumor targeting metal complex, wherein the ligand is one of a phenanthroline derivative, a dipyridyl derivative, a benzimidazole derivative, a phenyl-pyridine derivative and a thiophene-pyridine derivative; the targeting molecule is one of biotin, folic acid, integrin, transferrin, cell-penetrating peptide, octa-poly-L-arginine, MUC-1 attached membrane protein, galactosamine, neovascular targeting peptide and a granulocyte-macrophage colony-stimulating factor; the metal ions are ruthenium, osmium, rhodium or iridium ions. The complex obtained by the method provided by the invention can be selectively absorbed by tumor cells, and induces apoptosis of the tumor cells. In a nude mouse tumor-bearing model, the tumor targeting metal complex can image and treat tumors, and the toxicity of organs and tissues is reduced.
Owner:JINAN UNIVERSITY
Preparation and application of quantum dot-based multifunctional nano siRNA (Small Interfering Ribonucleic Acid) carrier system
ActiveCN103830745ATargetedGood biocompatibilityGenetic material ingredientsPharmaceutical non-active ingredientsTumor targetQuantum yield
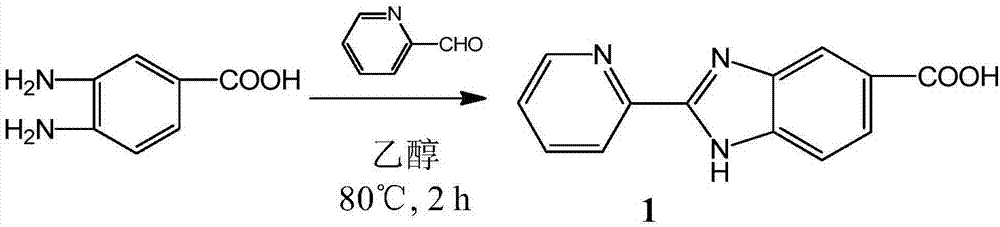
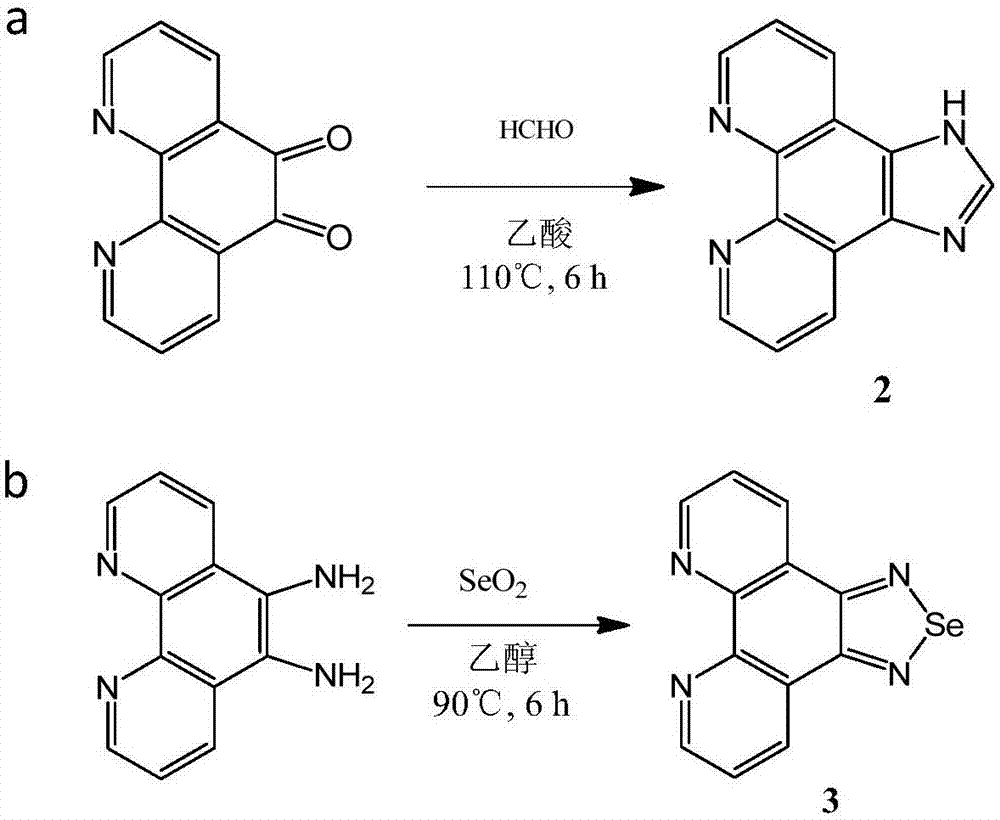
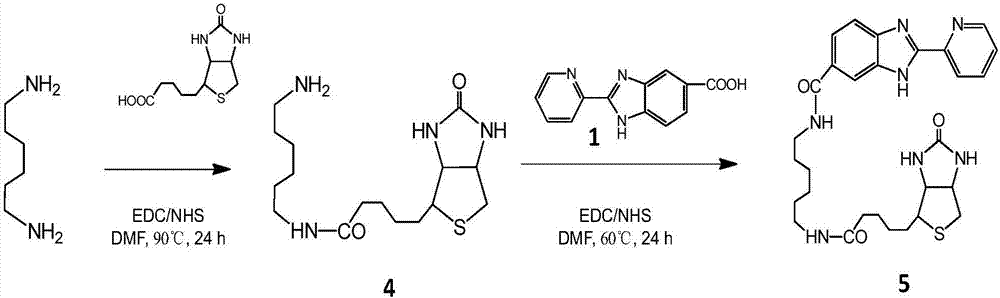
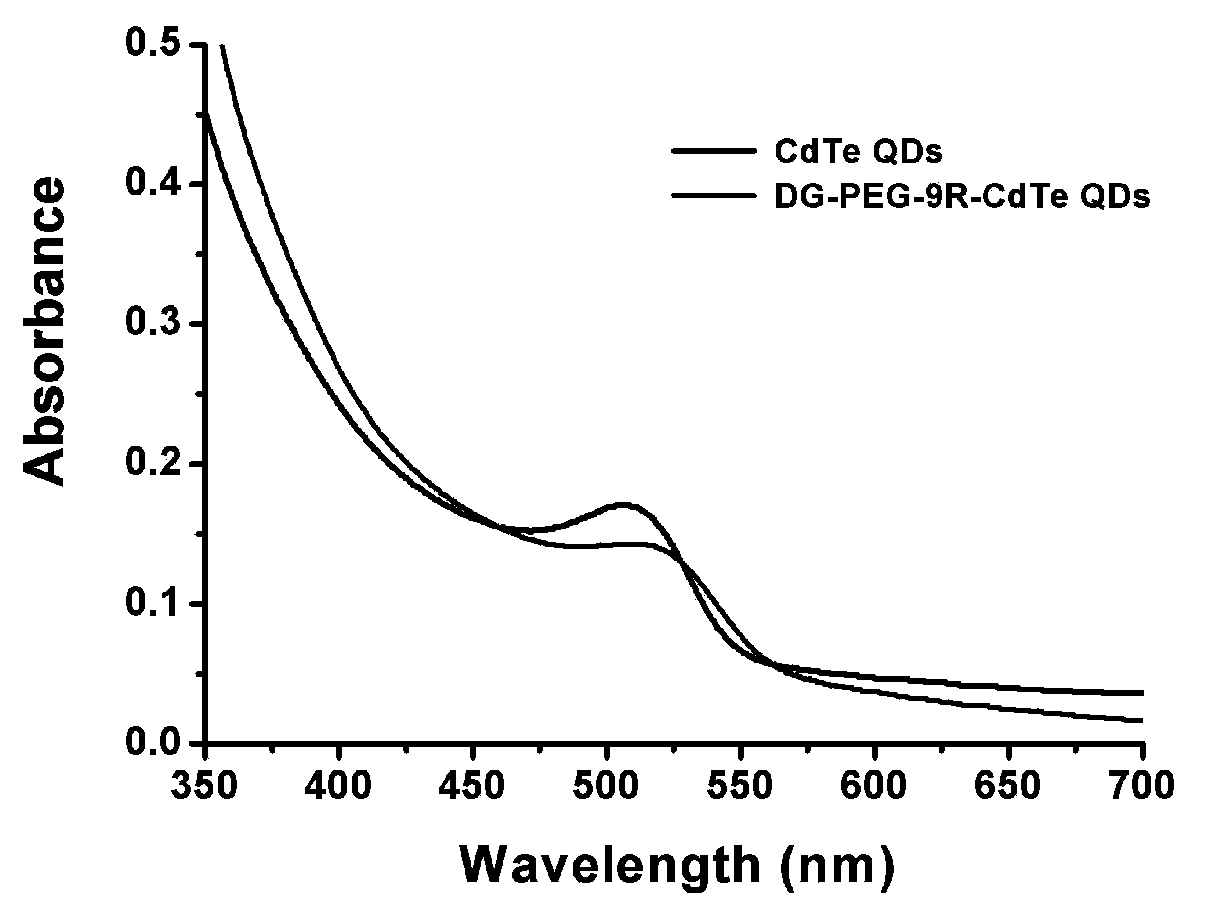
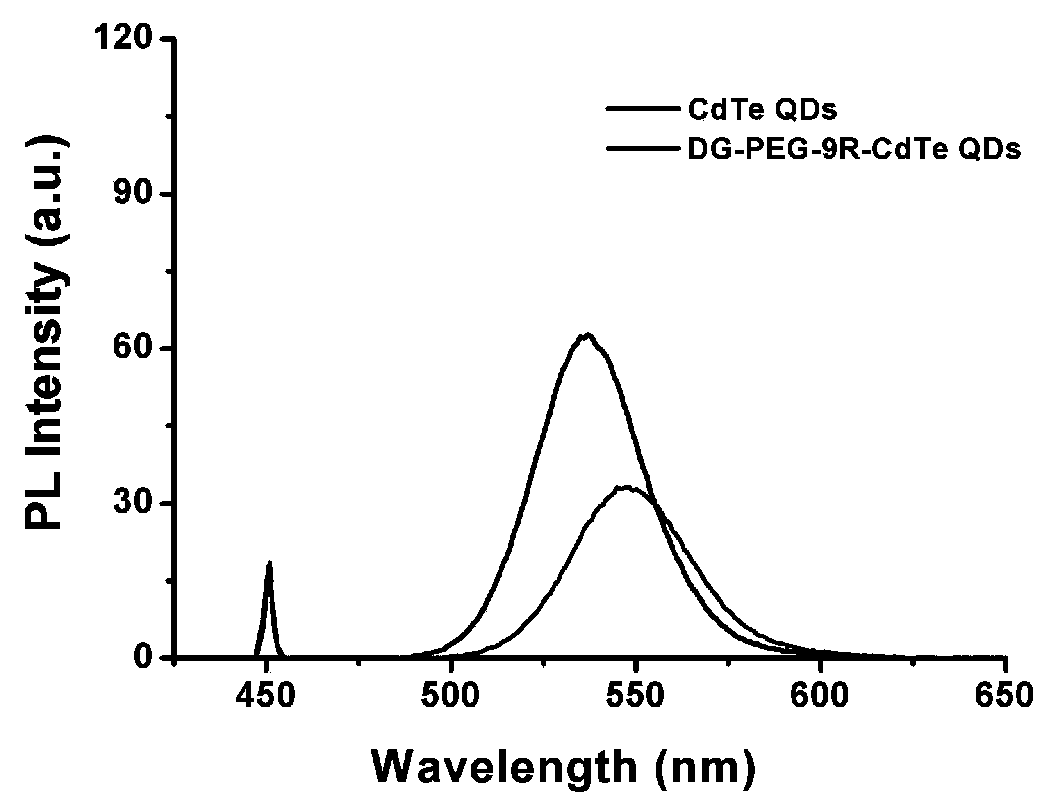
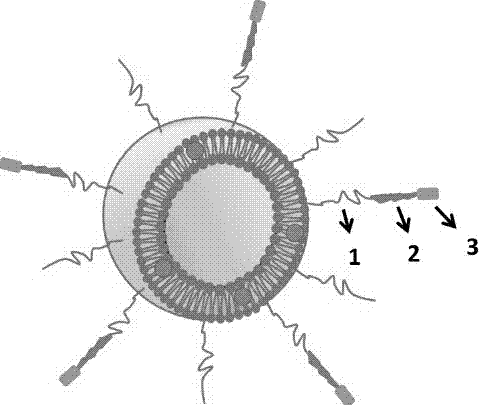
The invention relates to a preparation method and application of a multifunctional nano siRNA (Small Interfering Ribonucleic Acid) carrier system which integrates tumor targeting, pH sensitivity, visualization and the like. The core of the siRNA nano carrier system consists of cadmium telluride quantum dots. A nano carrier shell consists of a tumor targeted gene, a 9 poly-l-arginine peptide chain and PEG (Polyethylene Glycol). The siRNA nano carrier is good in biocompatibility, strong in tumor targeting and high in cell transfection efficiency, and has the characteristics of high quantum yield, strong optical stability, lossless in-position real-time monitoring and the like, so that the system is suitable for being used as a nano transmission system for in vivo gene therapy of tumors.
Owner:澎立生物医药技术(上海)股份有限公司
Double receptor identified serial cell-penetrating peptide modified tumor targeted nano drug delivery system
The invention provides a serial cell-penetrating peptide modified tumor targeted and tumor penetrated nano drug delivery system capable of simultaneously identifying an integrin receptor and a transmembrane protein receptor, wherein the serial cell-penetrating peptide is mainly formed by connecting a DGR polypeptide fragment covalently at the C-tail end of polyarginine of the cell-penetrating peptide. The polypeptide not only can selectively identify two highly expressed receptors on the surfaces of the tumor cells, but also can be efficiently mediated into the cells. The nano drug delivery system mainly consists of a nano carrier, a drug and a lipid-polyethylene glycol-serial cell-penetrating peptide ligand interstitial compound and is a potential tumor targeted treatment carrier.
Owner:SICHUAN UNIV
Chitosan based polymer conjugate and a method for producing the same
InactiveUS8431543B2Improve in vivo transfer efficiencyLow cytotoxicityPeptide/protein ingredientsGenetic material ingredientsPolyethylene glycolCytotoxicity
The present invention relates to a conjugate of chitosan and polyamine polymer that is useful for transferring a desired gene medicine into cells, and a method for preparing the same. In particular, the present invention relates to a double conjugate that is prepared by 1 in king poly-L-arginine to low molecular weight chitosan or triple conjugate that is prepared by additionally linking polyethylene glycol (PEG) to the double conjugate, and a method for preparing the same. The chitosan based cationic polymer conjugate of the present invention forms a complex with negatively charged gene medicine such as plasmid DNA and small interfering RNA to efficiently transfer the desired gene medicine into cells with low cytotoxicity. Accordingly, the conjugate can be used as an effective delivery system for in vivo administration of gene medicine.
Owner:ENGENE INC
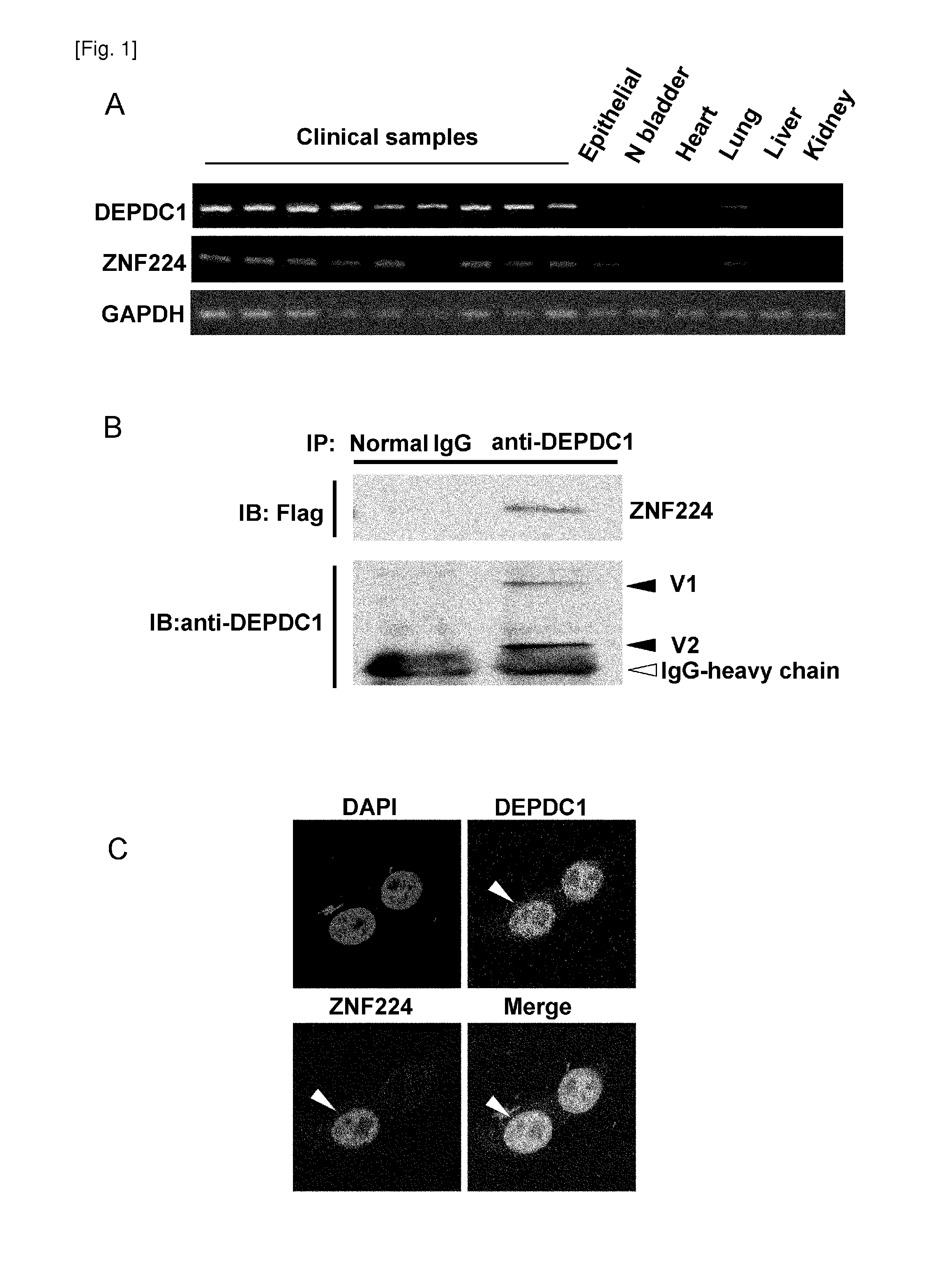
Method for treating or preventing bladder cancer using the depdc1 polypeptide
InactiveUS20110237518A1Improved prognosisIncrease probability recoveryOrganic active ingredientsPeptide/protein ingredientsCancer therapyAmino acid
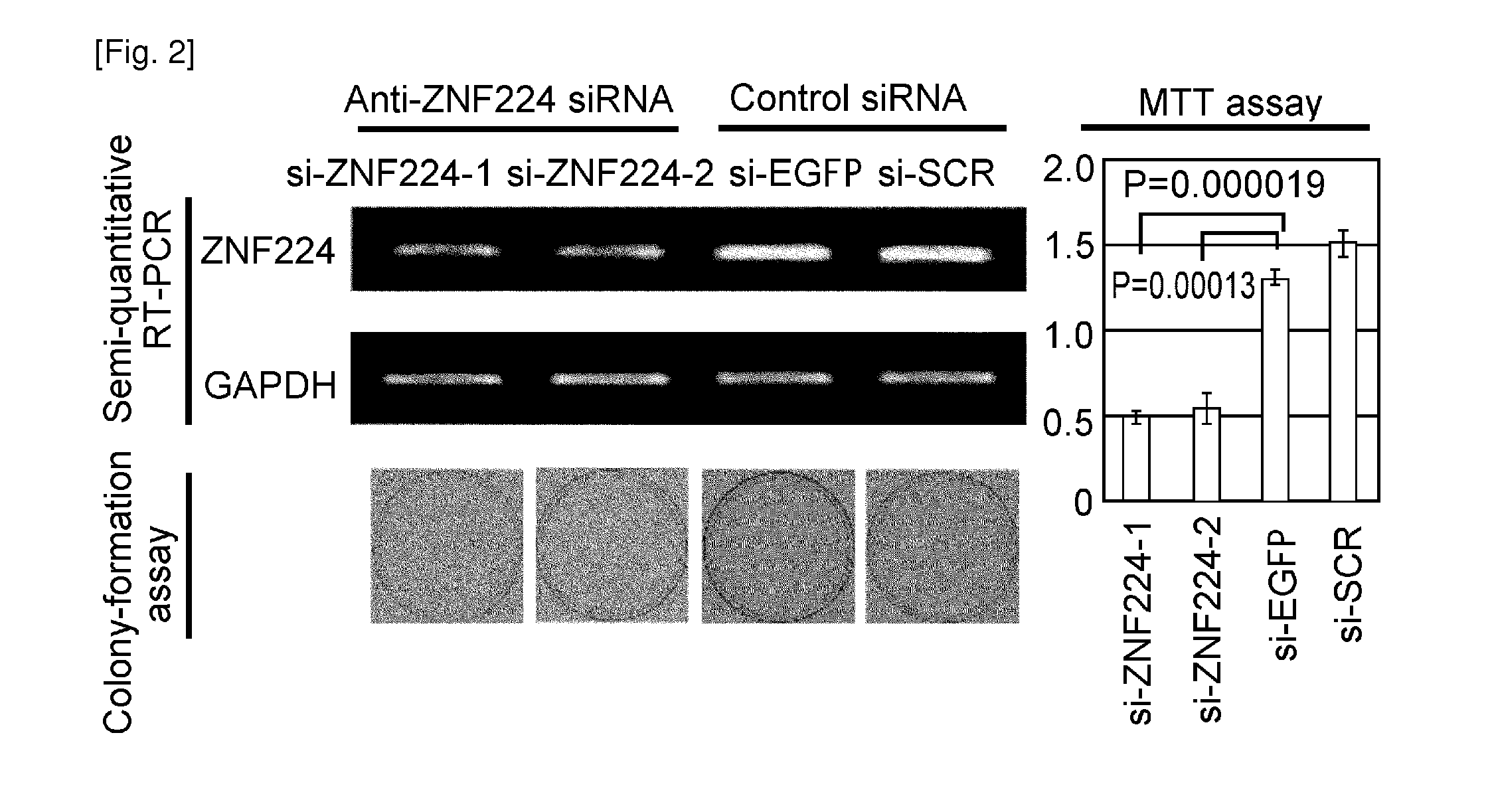
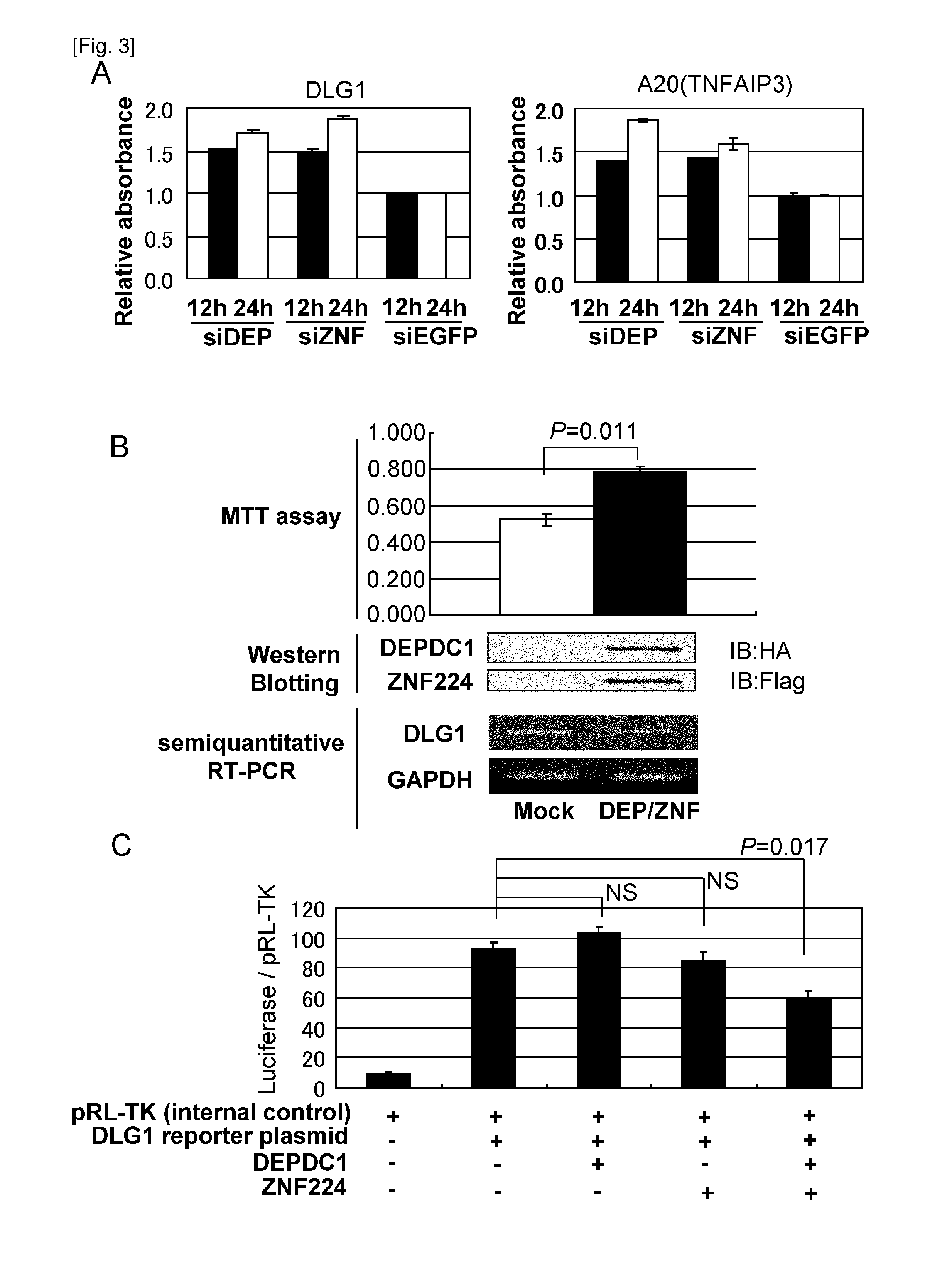
The present invention provides therapeutic agents and methods for treating cancer using the polypeptides posed of an amino acid sequence which includes a polypeptide fragment of DEPDC1. The polypeptides of the present invention can be introduced into cancer cells by modifying the polypeptides with transfection agents such as poly-arginine. Furthermore, the present invention provides methods of screening for therapeutic agents or compounds useful in inhibition of the DEPDC1 / ZN-F224 complex formation or the treatment of cancer. The present invention also provides siRNAs targeting the ZNF224 gene, which are suggested to be useful in the treatment of bladder cancer.
Owner:ONCOTHERAPY SCI INC
Rabies virus glycoprotein-derived peptide and application thereof
InactiveCN102174110AGood treatment effectMacromolecular non-active ingredientsHybrid peptidesDiseaseTherapeutic effect
The invention belongs to the field of biomedicine, and relates to a rabies virus glycoprotein-derived peptide and application thereof. The rabies virus glycoprotein-derived peptide is obtained by connecting the carboxyl terminal of a peptide fragment from a 189th position to a 214th position or a 330th position to a 357th position of a rabies virus glycoprotein with nine arginines through a connecting peptide, can quickly and specifically carry bioactive macromolecules such as proteins and the like to pass through a blood brain barrier so as to realize brain targeted medicine delivery, and can be used for preparing a brain targeted medicine delivery carrier; therefore, a high-efficiency, safe and non-invasive method is provided for the brain targeted delivery of the bioactive macromolecules and other active micromolecules which difficultly pass through the blood brain barrier, the treatment effect on brain diseases is enhanced, and the rabies virus glycoprotein-derived peptide has good development and application prospects in fields of research and development of new medicines for the brain diseases, and clinical treatment of the brain diseases.
Owner:SOUTHWEST UNIV
Formulations of factor VIIa inhibitors and utility
A nanoparticle, a chemical structure, and a treatment method for treating a patient having a disorder. The nanoparticle includes a poly L-arginine polymer and a Factor VIIa inhibitor conjugated to or encapsulated in the poly L-arginine polymer. The chemical structure includes a Factor VIIa inhibitor that includes at least one nitric oxide (NO) donor. The treatment method administers a therapeutic effective amount of the nanoparticle or chemical structure to the patient to treat the disorder. The disorder may be a vascular disorder, pulmonary hypertension, cardiac insufficiency, a neurological disorder, and combinations thereof.
Owner:MOUSA SHAKER A
Method and composition of glycosaminoglycans in sickle cell and vascular disorders
A composition that includes a nanoparticle. The nanoparticle includes a shell which encapsulates sulfated non-anticoagulant heparin (SNACH). The shell includes poly L-arginine. The SNACH is ionically or covalently bonded to the poly L-arginine. A method for treating a disorder of a subject includes: administering to the subject a therapeutically effective amount of the composition for treating the disorder. The disorder is a vascular disorder, a complication of the vascular disorder, or a combination thereof.
Owner:MOUSA SHAKER A
Delivery molecule and nanoparticle, preparation method and application
InactiveCN108424437AImprove delivery efficiencyOrganic active ingredientsPowder deliveryNucleotideNanoparticle
The invention provides a delivery molecule and a nanoparticle, a preparation method and application. The delivery molecule comprises cell penetrating peptide, poly-L-arginine is linked to the C or N end of the cell penetrating peptide, the delivery molecule can combine with siRNA and other nucleotides, protein and DNA to form a nanoparticle with a diameter of 600nm, the delivery molecule can deliver siRNA into cells, and release siRNA to play a therapeutic role. The invention indicates that the delivery molecule can deliver transferred substances into cells or animal bodies both on the cell and animal level and release the transferred substances to play a part, and the delivery molecule is non-toxic and has high delivery efficiency.
Owner:QINGDAO AGRI UNIV
Agent for enhancing the resistance of liposome against biological component, and liposome modified with the agent
InactiveUS20100104623A1Improve the immunityImprove delivery capabilitiesPeptide/protein ingredientsPeptidesLipid formationAdditive ingredient
It is intended to provide a positively charged liposome, particularly having a polyarginine peptide on a surface thereof, which is capable of increasing the resistance to a negatively charged biological component such as a protein in the blood and maintaining a high ability to deliver a substance even in the blood. An agent for enhancing the resistance of liposome against biological component comprising a peptide having at least one of the following characteristics (a) and (b) as an active ingredient:(a) A peptide comprising an amino acid sequence represented by SEQ ID No: 1 or 2; and(b) A peptide comprising an amino acid sequence represented by SEQ ID No: 1 or 2, in which one or more amino acids are deleted, substituted or added, and having an activity for promoting lipid membrane fusion under acidic condition. An agent for enhancing the resistance of liposome against biological component in this invention, being included in a liposome, is capable of increasing the resistance to a negatively charged biological component such as a protein in the blood and maintaining a high ability to deliver a substance even in the blood.
Owner:HOKKAIDO UNIVERSITY
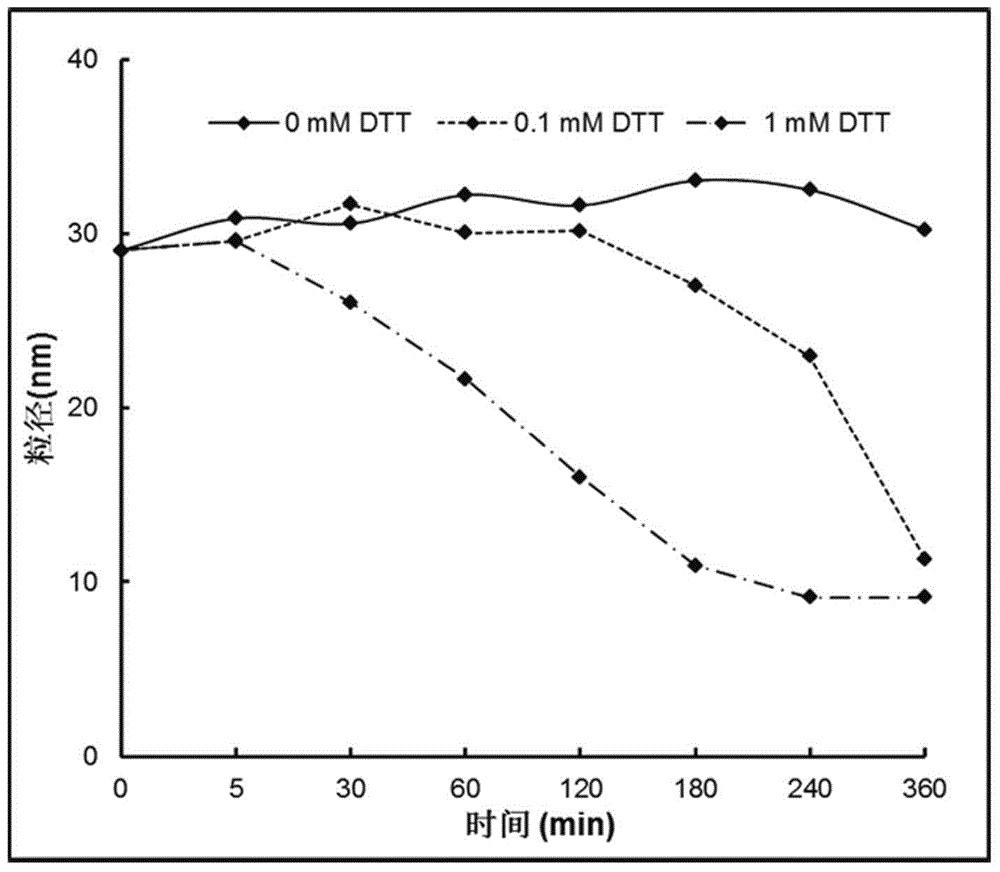
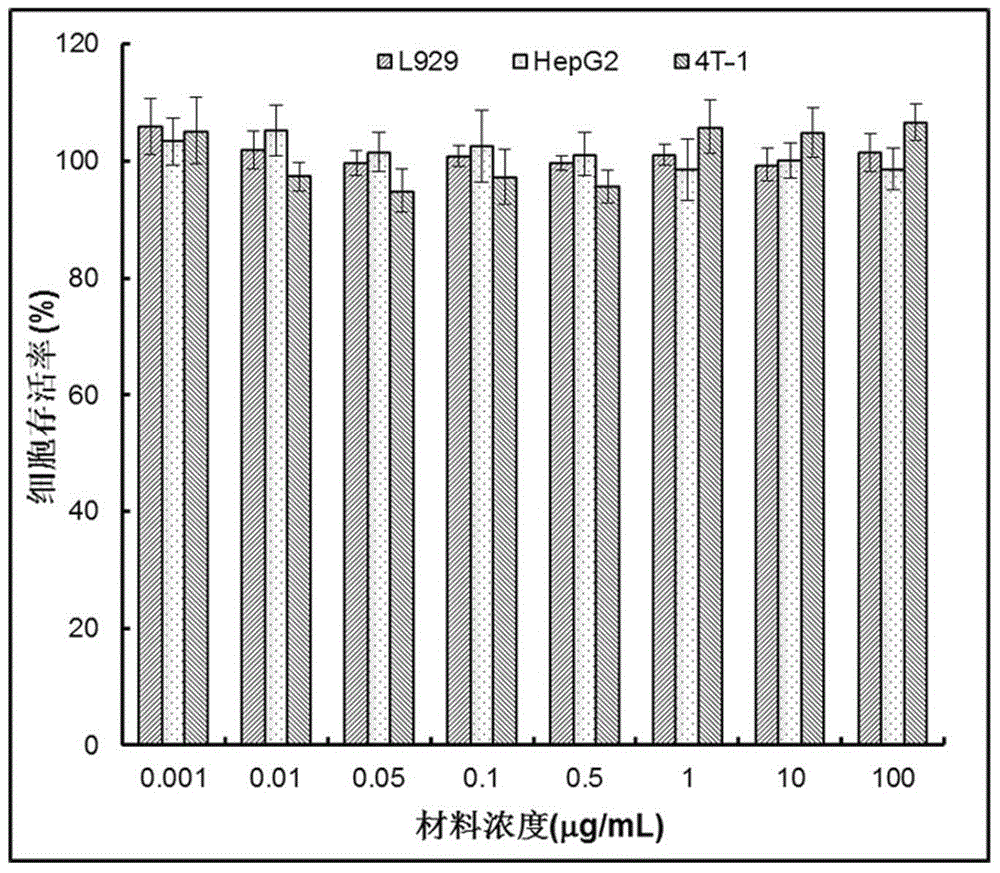
Reduced sensitive type polymer with effect of arginine membrane penetration as well as preparation method and application of reduced sensitive type polymer
ActiveCN105037739APromote aggregationSmall toxicityPeptide/protein ingredientsGenetic material ingredientsCancer cellCell membrane
The invention provides a reduced sensitive type polymer with the effect of arginine membrane penetration as well as a preparation method of the reduced sensitive type polymer. The polymer can self-assemble in an aqueous medium to form micelle of a shell-core structure; the polymer contains a poly-L-arginine structure, so that the speed that the polymer penetrates through a cell membrane to enter cancer cells is increased; meanwhile, the polymer contains a disulfide bond, so that the polymer can be degraded by relatively high reduction potential energy in cancer cells. The invention further provides application of the reduced sensitive type polymer with the effect of arginine membrane penetration as a drug carrier. After being gathered at tumor tissue through the enhanced permeability and retention effect, the carrier can quickly enter the cancer cells, so that bioavailability and the treatment effect of the drug can be improved.
Owner:SICHUAN UNIV
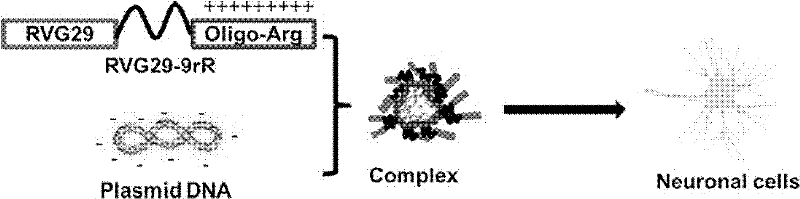
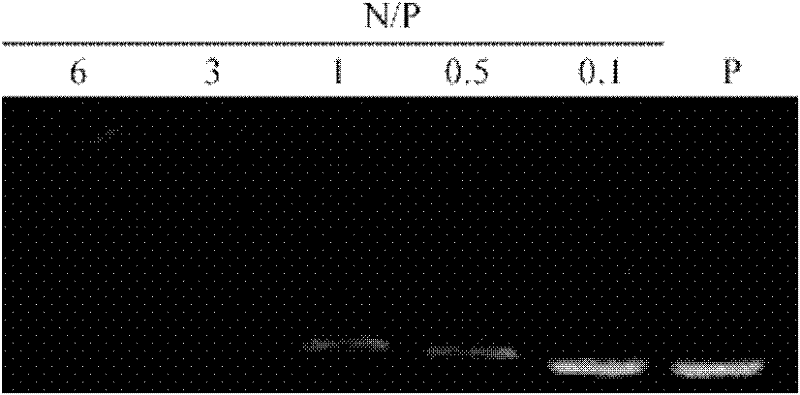
Peptide capable of transporting DNA to target neurocytes and brain
InactiveCN102250222ASolving difficulties that are difficult to be transported to the brain and expressedSmall sizeDepsipeptidesMacromolecular non-active ingredientsAcetylcholine receptorDrug carrier
The invention discloses a peptide capable of transporting a DNA to target neurocytes and brain and belongs to the field of biological science and medicine carriers. The peptide is formed by combining a target sequence, a connecting sequence and a DNA combining sequence, wherein the target sequence is an acetylcholine receptor identifying ligand; and the DNA combining sequence is 9-poly-L-arginine, and the amino acid sequence of the 9-poly-L-arginine is represented by SEQ ID No.1 in a sequence list. The peptide can transport the DNA to the target neurocytes and brain.
Owner:HUAZHONG UNIV OF SCI & TECH
Compositions and method for Anti-sickling of red blood cells in sickle cell disease
A nano-composition that includes nanoparticles, a method of forming the nano-composition, and a method of using the composition. The nanoparticles include a polycationic polymer ionically bonded to one or more polyanionic Glycosaminoglycans (GAGs), wherein the polycationic polymer is chitosan, methylated chitosan, poly L-Lysine, or poly L-Arginine.
Owner:MOUSA SHAKER A
Arborescent polypeptide for conducting cell therapy on HPV infection and screening method, preparation method and application of arborescent polypeptide
InactiveCN106084008AStrong specificityImprove bindingViral antigen ingredientsAntiviralsResponse sensitivityScreening method
The invention relates to an arborescent polypeptide for conducting a cell therapy on HPV infection. The arborescent polypeptide is characterized by being formed by connecting four carboxyl groups of a C-terminal of an HPV epitope peptide with three amino groups of a poly-L-arginine framework composed of arginine residues, wherein an amino acid sequence of the epitope peptide is Tyr-Met-Leu-Asp-Leu-Gln-Pro-Glu-Thr-Gly-Gly-Gly. The invention further provides a screening method, a preparation method and application of the arborescent polypeptide. Through design and analysis of multiple kinds of combined bioinformatic software, the capacity of finding a new epitope is improved, the epitope loading orientations achieve consistency, and the specific combination capacity and reaction sensitivity of the epitope peptide are exponentially improved in unit space. The epitope peptide is single in structure and property and has the great advantages of being high in specificity, free of potential biological hazards and capable of achieving stardardization in preparation process.
Owner:江苏安泰生物技术有限公司
Telmisartan enteric-coated tablets and preparation method thereof
ActiveCN109350604AImprove solubilityLow hygroscopicityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityMoisture absorption
The invention belongs to the field of pharmaceutical preparations, and particularly relates to telmisartan enteric-coated tablets and a preparation method thereof. The telmisartan enteric-coated tablets are each composed of a tablet core, an isolating layer and an enteric coating. The table cores are composed of, by weight, 20-40 parts of telmisartan, 8-20 parts of poly-L-arginine, 10-60 parts ofmannitol, 60-160 parts of microcrystalline celluloses, 3-9 parts of povidone and 0.5-3 parts of magnesium stearate. Poly-L-arginine is adopted for alkalizing telmisartan, in this way, the solublenessof telmisartan can be improved remarkably, and moisture absorption is reduced; meanwhile, mannitol is added, in this way, powder with good fluidity can be obtained, and the prepared tablets are high in dissolution rate and good in stability; common rapid-disintegration oral administration preparations are prepared into the enteric-coated tables, in this way, it can be avoided that the preparationsdisintegrate in gastric acid, and telmisartan is separated out, the bioavailability of telmisartan is improved remarkably, and application prospects are good.
Owner:广州迈凯安生物医药研究院有限公司
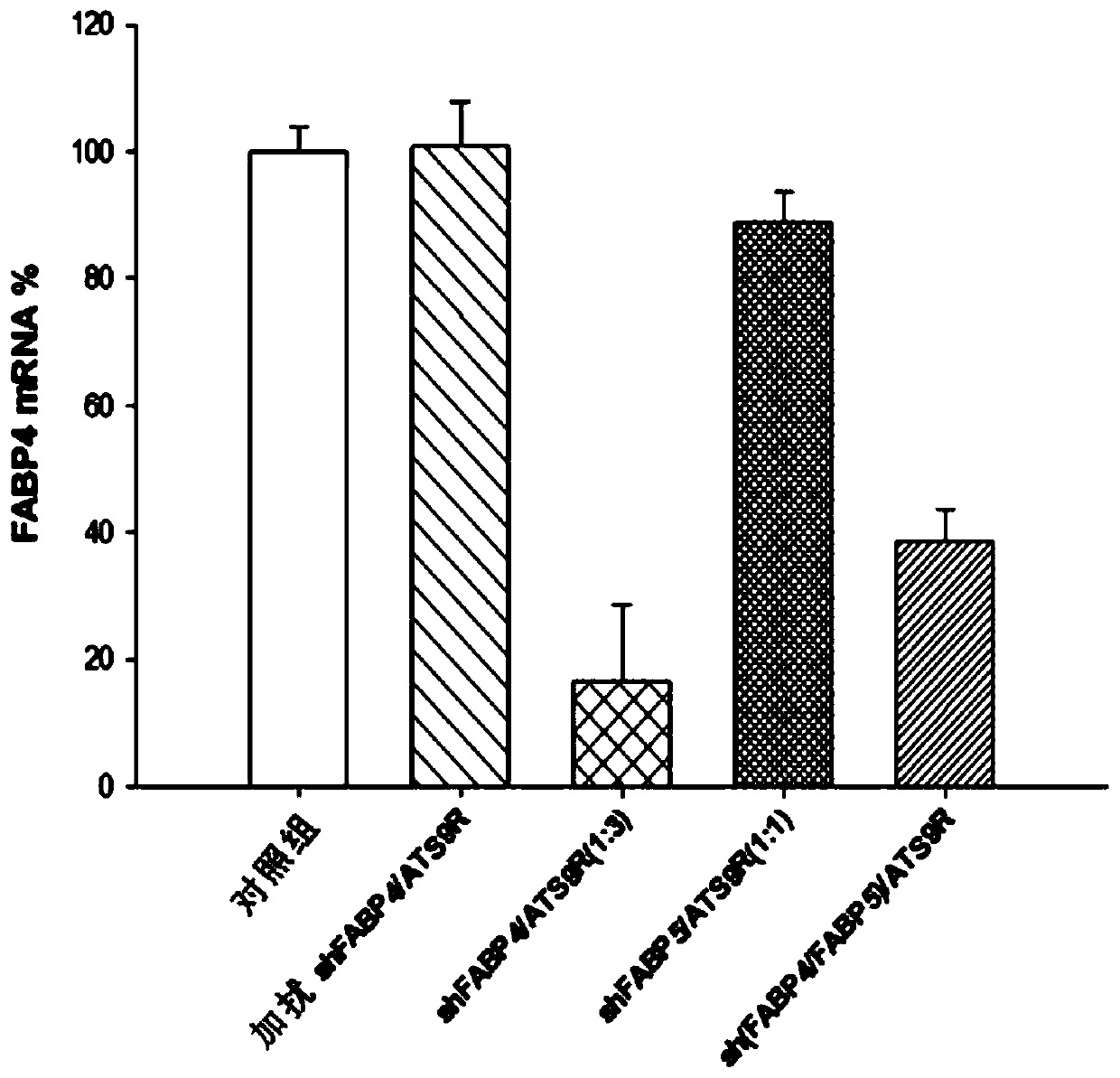
Adipocyte-targeting non-viral gene delivery complex comprising dual plasmid vector
ActiveCN109844122ANo cytotoxicityExcellent obesity treatment effectOrganic active ingredientsVectorsGene deliveryMedicine
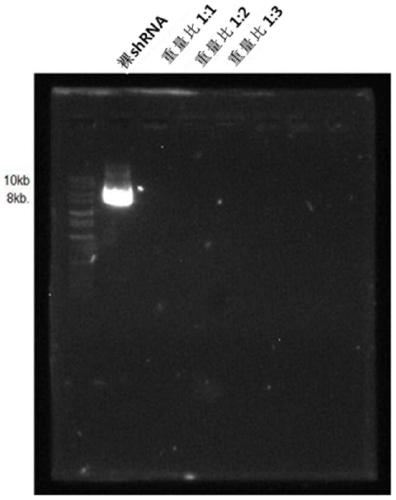
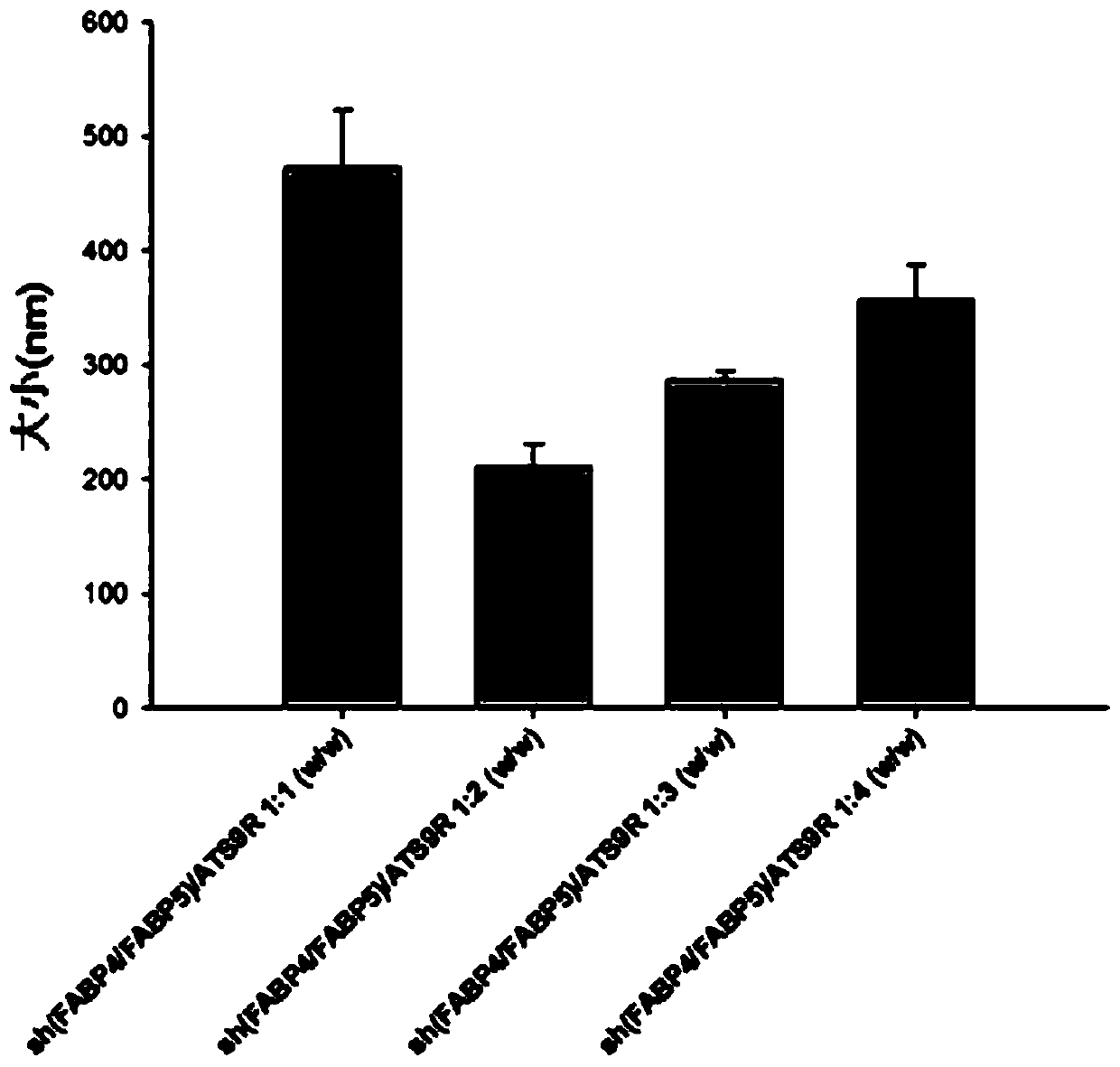
The present invention relates to: an adipocyte-targeting non-viral gene delivery complex comprising a sh(FABP4 + FABP5) dual plasmid vector; and treatment for obesity and obesity-induced metabolic syndromes by using the same and, more particularly, to a gene delivery complex comprising: an adipocyte-targeting sequence; an arginine 9 (R9) peptide; and a dual plasmid vector comprising a gene for treatment of obesity and obesity-induced metabolic syndromes, wherein the gene for treatment of obesity and obesity-induced metabolic syndromes is a base sequence inhibiting the expression of a FABP4 gene and a FABP5 gene. According to the present invention, in order to treat obesity-related diseases, a dual plasmid vector capable of simultaneously inhibiting the FABP4 and FABP5 genes is produced, and since a gene delivery complex is provided by binding the vector to a predetermined delivery system specifically delivering the same to adipocytes, an excellent and non-toxic obesity treatment effecttargeting only adipocytes can be achieved.
Owner:IUCF HYU (IND UNIV COOP FOUND HANYANG UNIV)
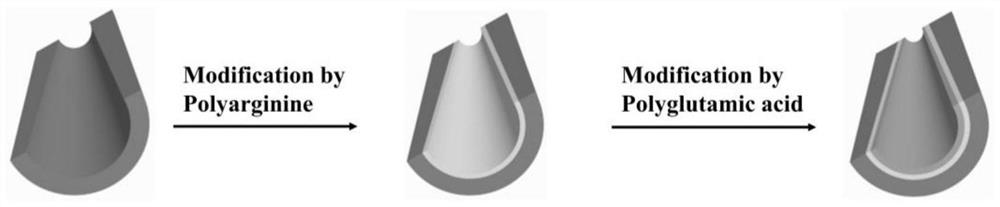
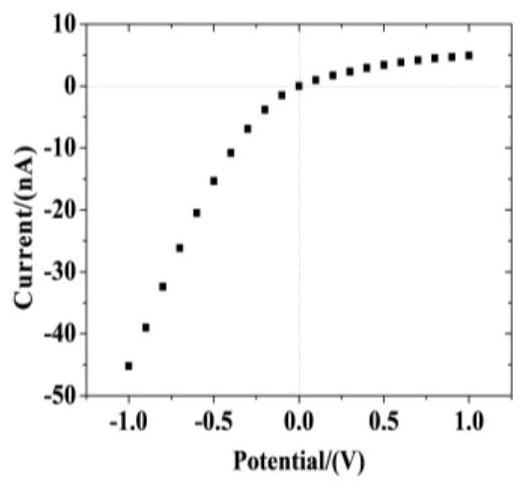
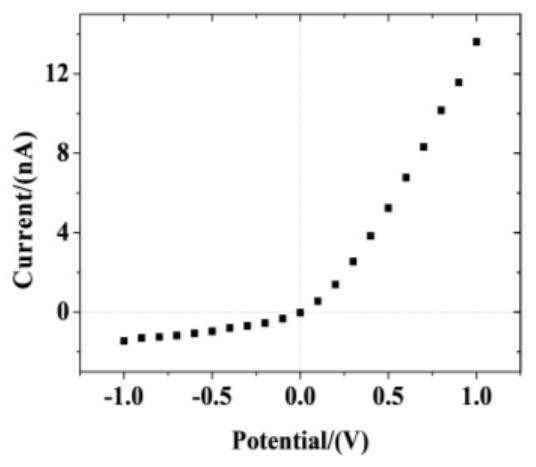
Method for preparing pH-responsive nanofluidic diode based on nanopores modified by polyarginine and polyglutamic acid layer by layer
The invention relates to a method for preparing a pH-responsive nanofluidic diode based on nanopores modified by polyarginine and polyglutamic acid layer by layer. The inner surfaces of the nanoporesare modified by adopting a layer-by-layer polyelectrolyte electrostatic adsorption method. The method comprises the following steps of firstly, carrying out chemical etching on a PET film to obtain asingle conical polyarginine modified nanopore, and enabling the nanopore modified by the polyarginine molecule to be subjected to pH action to obtain the nanofluidic diode with anion selectivity. Polyglutamic acid molecules have a large number of carboxyl groups, polyarginine and polyglutamic acid can be modified on a polyarginine molecular layer through an electrostatic adsorption method, and nanopores modified by polyarginine and polyglutamic acid layer by layer are obtained. The nanopores modified by polyarginine and polyglutamic acid layer by layer are subjected to pH action to obtain thenanofluidic diode with cation selectivity.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Nano preparation for combined transportation of nucleic acids and polypeptides and preparation method
ActiveCN105327358AAvoid toxicityTaking into account stabilityOrganic active ingredientsPowder deliveryHydrogenNanoparticle
The invention aims to overcome defects that bio-macromolecular nucleic acid and polypeptide medicines degrade easily in bodies and can hardly reach target cells and provides a nano preparation for combined transportation of nucleic acids and polypeptides and a preparation method. According to the nano preparation, multifunctional cationic polypeptides and cationic liposomes / nucleic acid non-viral carriers which are safe to human bodies are adopted to coat polypeptide medicines and nucleic acid medicines simultaneously to form nanoparticles, and bio-macromolecular medicine carriers capable of treating diseases through combination of the polypeptides and the nucleic acids are prepared. The cationic liposomes and the polypeptides are mixed and then assembled with the nucleic acid medicines under the electrostatic effect, the nanoparticles with positive charges on surfaces are formed, and nano target particles with negative charges on surfaces are further formed under the electrostatic effect by covering hyaluronic acids with hydrogen bonds. The nano preparation has the advantages that the polypeptide medicines and the nucleic acid medicines are loaded simultaneously, the polypeptide medicines are released continuously through breakage of degradable bonds between the polypeptide medicines and poly-L-arginine, and the nucleic acids are released effectively through disassembly of the carriers.
Owner:SHANGHAI JIAO TONG UNIV
Degradable organic and inorganic composite nano-particle capable of realizing anti-tumor treatment, and preparation method thereof
ActiveCN107982242AGood treatment effectEfficient release effectOrganic active ingredientsEnergy modified materialsThermal stimulationBiocompatibility Testing
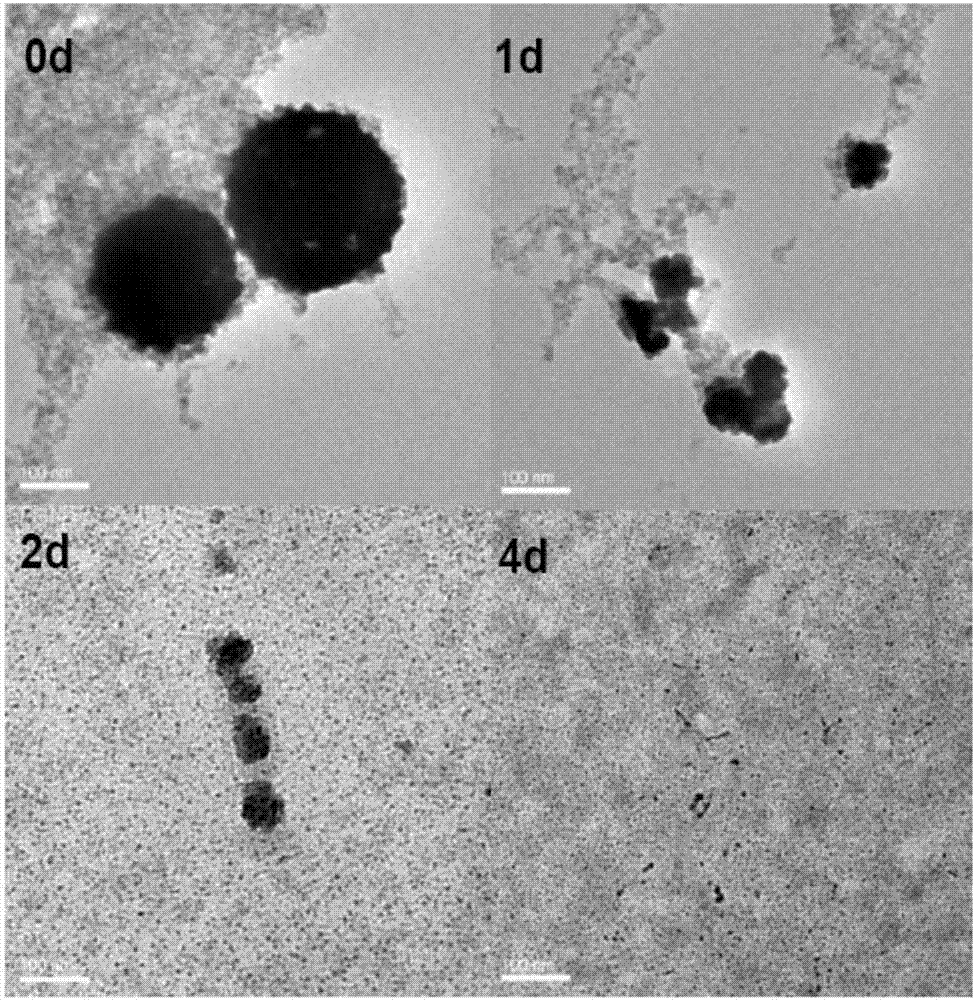
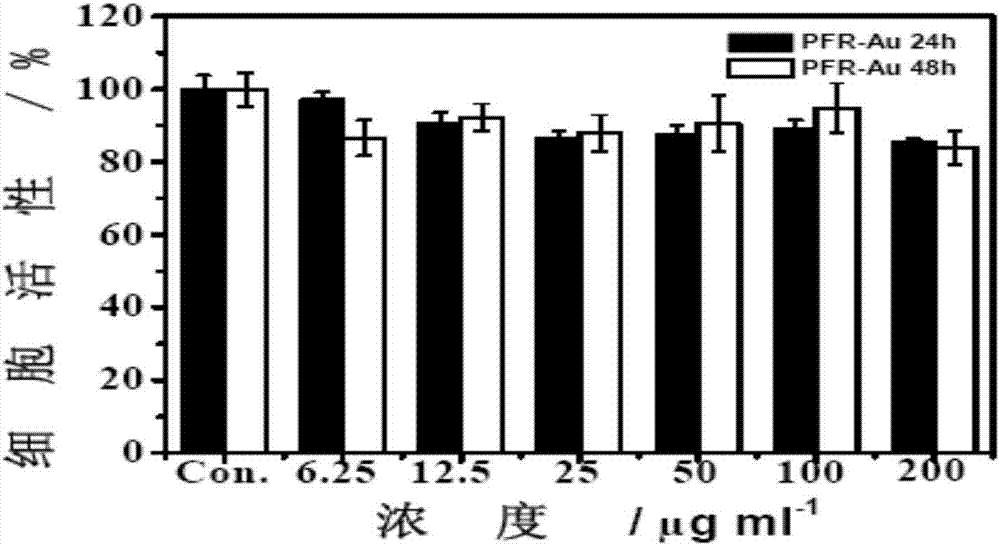
The invention discloses a degradable organic and inorganic composite nano-particle capable of realizing anti-tumor treatment, and a preparation method thereof. The inner part of the particle is Fe3O4,the middle layer of the particle is gamma-PGA (polyglycolic acid) and Poly-l-arginine (PA), and the outer layer is a golden shell. The size of the particle is about 200nm, and the particle has biodegradability. The preparation method for the degradable organic and inorganic composite nano-particle comprises the following steps: through a gamma-PGA, Fe<2+> and Fe<3+> coprecipitation method, preparing high-dispersion magnetic Fe3O4 nano-particles; then, through a static electricity mutual function, adsorbing doxorubicin hydrochloride (DOX.HCl) and PA to the surface of the particle; finally, carrying out in-situ reduction of chloroauric acid on the surface of the PA to prepare the gamma-PGA-Fe3O4-DOX-PA@Au(PFDR-Au) composite nano-particles. The method has the advantages of simpleness in operation, short time consumption, small material consumption, no organic solvent, moderate and controllable synthesis condition and high reproducibility. The nano-particle has biodegradability and good biocompatibility, Adriamycin can be released under the condition of photo-thermal stimulation and micro acid, and a tumor growth inhibition effect is obvious.
Owner:BEIJING UNIV OF CHEM TECH
NOVEL FORMULATIONS OF FACTOR VIIa INHIBITORS AND UTILITY
A method for treating a subject, such as a human patient, having a vascular disorder. The treatment method administers a therapeutic effective amount of a nanoparticle or a chemical structure to the subject to treat the disorders. The nanoparticle includes a poly L-arginine polymer and a Factor VIIa inhibitor conjugated to, or encapsulated in, the poly L-arginine polymer. The chemical structure includes a Factor VIIa inhibitor that includes at least one nitric oxide (NO) donor. The disorder may be sickle cell disease; stimulated or pathological angiogenesis associated disorders, cancer, ocular angiogenesis-mediated disorders such as diabetic retinopathy and macular degeneration, coagulation and / or platelet activation-associated disorders, pulmonary hypertension, or combinations thereof.
Owner:MOUSA SHAKER A
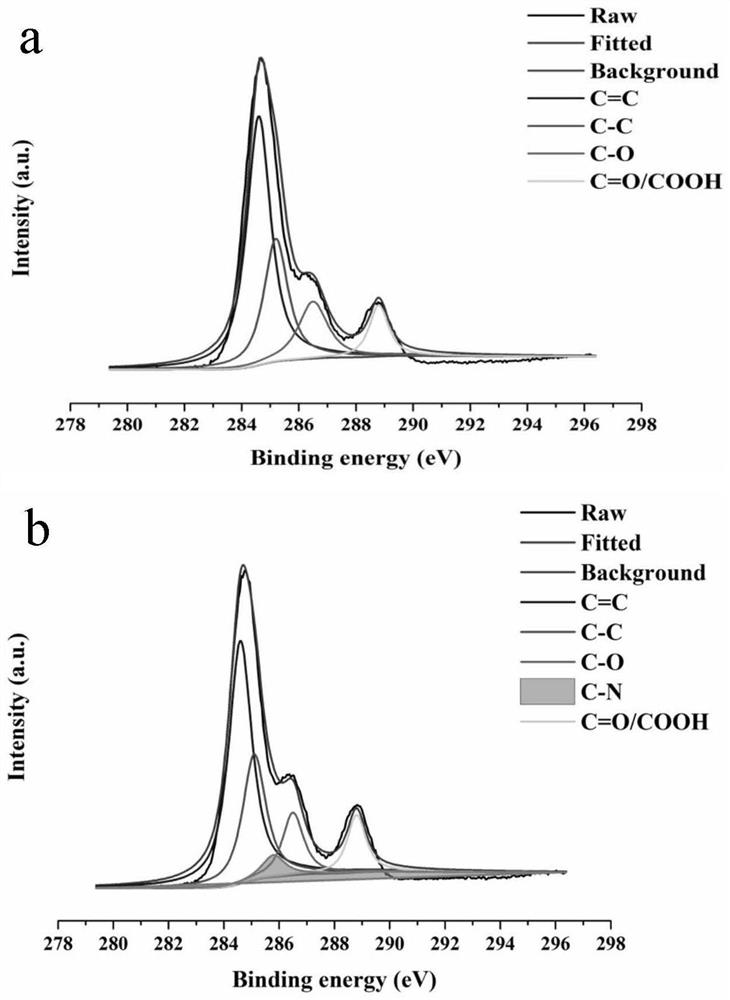
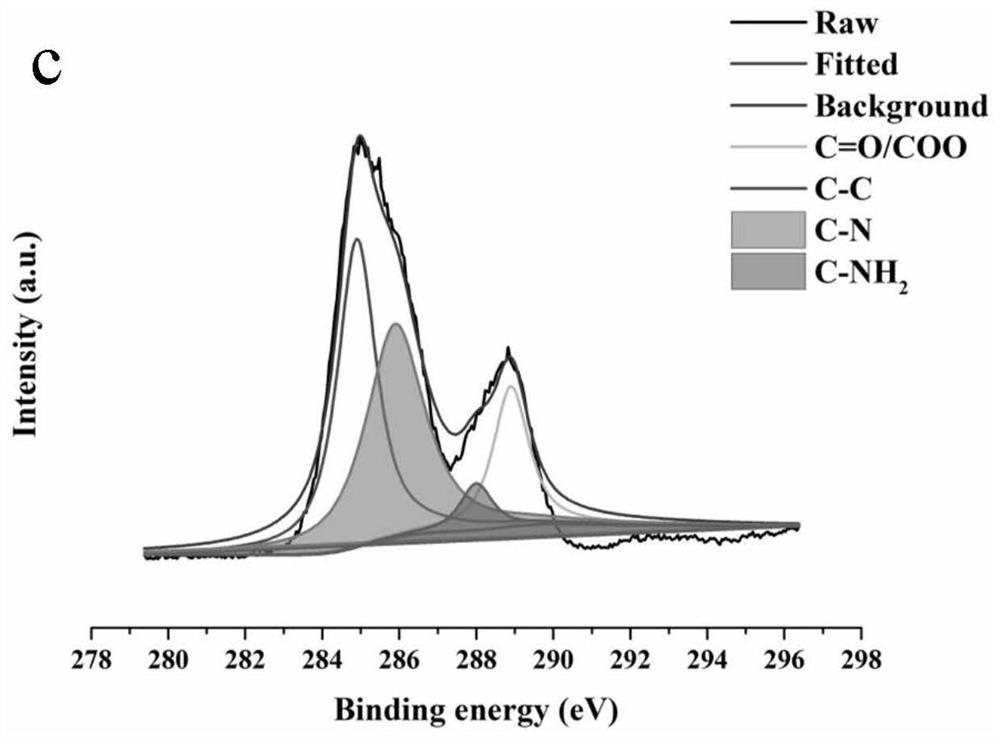
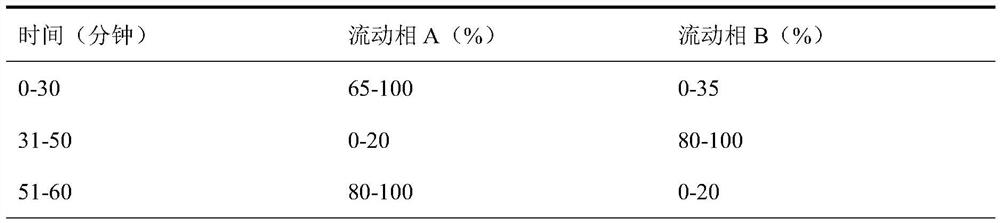
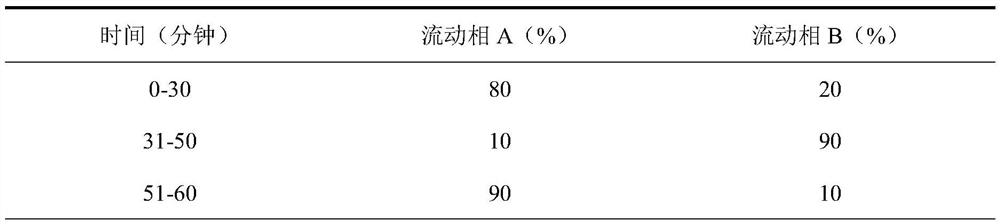
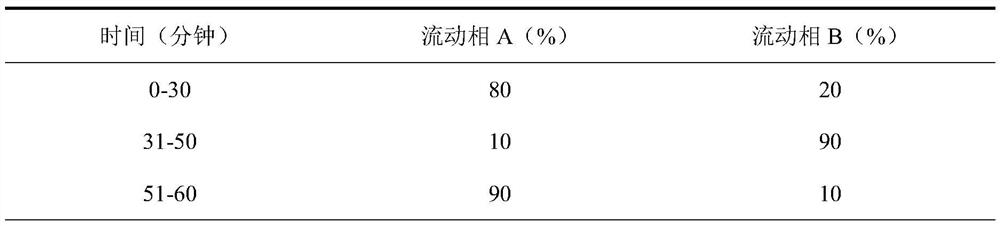
Analysis method for detecting impurity in aspartate ornithine
The invention discloses an analysis method for detecting impurities in aspartate ornithine and belongs to the field of analytical chemistry. The analysis method comprises the following steps: by usingliquid chromatography, under the conditions that an amino bonded silica gel filling agent, namely Thermo APS-2 of 4.6 mm*250 mm*5 [mu]m, is adopted, 0.1 mol / L monopotassium phosphate buffer solution-acetonitrile (35:65) is used as a mobile phase A, 0.1 mol / L monopotassium phosphate buffer solution-acetonitrile (50: 50) is used as a mobile phase B, a detection wavelength is 200 nm, a flowing speedis 1.0 ml / minute, and a column temperature is 30 DEG C, performing gradient elution, and performing analysis. By adopting the technical scheme, impurities such as 3-amino-2-piperidone, di-poly-l-arginine and an alpha-aspartate biopolymer and an aspartate condensation compound can be rapidly and accurately qualitatively and quantitatively analyzed, a good separation degree can be achieved, a relatively low detection limit and quantitative limit can be achieved, a relatively wide linear range can be achieved, the contents of different impurities can be relatively accurately calculated, and technical support can be provided for further monitoring and studying impurities in the aspartate ornithine.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Transferring carrier of liver cancer target to ward gene introduced by acceptor of alphafetoprotein enhancement type epidermal growth factor dual controlled by transcription and transfer
A both transcrption and transfer controlled, alpha-fetoprotein enhanced, and epidermal growth factor receptor mediated liver cancer target gene transfer carrier is composed of alpha-fetoprotein mediated exogenous DNA, ligand oligopeptide, polycation polypeptide and endocytic glubule release oligopeptide. It can suppress the growth of liver cancer cells.
Owner:SHANDONG UNIV
Rabies virus glycoprotein-derived peptide and application thereof
InactiveCN102174110BGood treatment effectMacromolecular non-active ingredientsHybrid peptidesDiseaseTherapeutic effect
The invention belongs to the field of biomedicine, and relates to a rabies virus glycoprotein-derived peptide and application thereof. The rabies virus glycoprotein-derived peptide is obtained by connecting the carboxyl terminal of a peptide fragment from a 189th position to a 214th position or a 330th position to a 357th position of a rabies virus glycoprotein with nine arginines through a connecting peptide, can quickly and specifically carry bioactive macromolecules such as proteins and the like to pass through a blood brain barrier so as to realize brain targeted medicine delivery, and can be used for preparing a brain targeted medicine delivery carrier; therefore, a high-efficiency, safe and non-invasive method is provided for the brain targeted delivery of the bioactive macromolecules and other active micromolecules which difficultly pass through the blood brain barrier, the treatment effect on brain diseases is enhanced, and the rabies virus glycoprotein-derived peptide has good development and application prospects in fields of research and development of new medicines for the brain diseases, and clinical treatment of the brain diseases.
Owner:SOUTHWEST UNIV
Formulations of Factor VIIa inhibitors and utility
A method for treating a subject, such as a human patient, having a vascular disorder. The treatment method administers a therapeutic effective amount of a nanoparticle or a chemical structure to the subject to treat the disorders. The nanoparticle includes a poly L-arginine polymer and a Factor VIIa inhibitor conjugated to, or encapsulated in, the poly L-arginine polymer. The chemical structure includes a Factor VIIa inhibitor that includes at least one nitric oxide (NO) donor. The disorder may be sickle cell disease; stimulated or pathological angiogenesis associated disorders, cancer, ocular angiogenesis-mediated disorders such as diabetic retinopathy and macular degeneration, coagulation and / or platelet activation-associated disorders, pulmonary hypertension, or combinations thereof.
Owner:MOUSA SHAKER A
Graphene polymer electrochemical sensor, preparation method and application of graphene polymer electrochemical sensor in detection of p-nitrophenol
ActiveCN113447545ALow detection limitWide linear detection rangeMaterial electrochemical variablesPhysical chemistryNitrophenol
The invention discloses a graphene polymer electrochemical sensor, a preparation method and application of the graphene polymer electrochemical sensor in detection of p-nitrophenol, and the preparation method comprises the following steps of carrying out primary electrochemical treatment on a carbon-based electrode to synthesize graphene in situ on the surface of the carbon-based electrode, and then carrying out secondary electrochemical treatment on the carbon-based electrode with the graphene synthesized on the surface, polycondensating the arginine into poly-arginine, and compounding the poly-arginine with the graphene. The graphene polymer electrochemical sensor provided by the invention is low in sensing detection limit, wide in linear detection range, high in stability and low in cost, can be used for preparing a disposable pNP test strip, and can also be used for in-situ long-time continuous monitoring of pNP.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
A kind of analytical method for detecting aspartic acid ornithine impurity
The invention discloses an analysis method for detecting impurities in aspartic acid ornithine, which belongs to the field of analytical chemistry; the method adopts liquid chromatography, amino-bonded silica gel filler, Thermo APS‑2 4.6×250mm, 5 μm, and 0.1mol / L potassium dihydrogen phosphate buffer-acetonitrile (35:65) is mobile phase A, 0.1mol / L potassium dihydrogen phosphate buffer-acetonitrile (50:50) is mobile phase B; the detection wavelength is 200nm; The flow rate is 1.0ml per minute; the column temperature is 30°C, and gradient elution is used for analysis. This technical solution can quickly and accurately detect 3‑amino‑2‑piperidone and dimer arginine in impurities aspartic acid ornithine acid, α-aspartic acid dimer, and aspartic acid condensate for qualitative and quantitative analysis, and the resolution is good, the detection limit and quantification limit are low, and the linear range is wide, so that it can be calculated more accurately The content of different impurities provides technical support for further monitoring and research of impurities in ornithine-aspartate.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com