Analysis method for detecting impurity in aspartate ornithine
A technique for aspartic acid ornithine and analytical methods, applied in the field of analytical chemistry, can solve the problem that there is no clear collection of quantitative and qualitative methods, and there is no disclosure of dimeric arginine and α-aspartic acid dimer gates Problems such as qualitative and quantitative analysis methods of aspartic acid condensates to achieve good resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Resolution detection
[0049] 1). Specific experimental operations:
[0050] Positioning solution of each impurity reference substance: accurately weigh out 10 mg of 3-amino-2-piperidone, α-aspartic acid dimer, aspartic acid condensate, and dimer arginine, and put them into 50 ml measuring bottles. In the medium, add mobile phase B to dissolve and dilute to the mark, shake well, and get ready.
[0051] Positioning solution for the test product: accurately weigh 100 mg of ornithine hydrochloride, aspartic acid, and ornithine aspartate, respectively, put them in a 10 ml measuring flask, add mobile phase B to dissolve and dilute to the mark, shake well, that is Got.
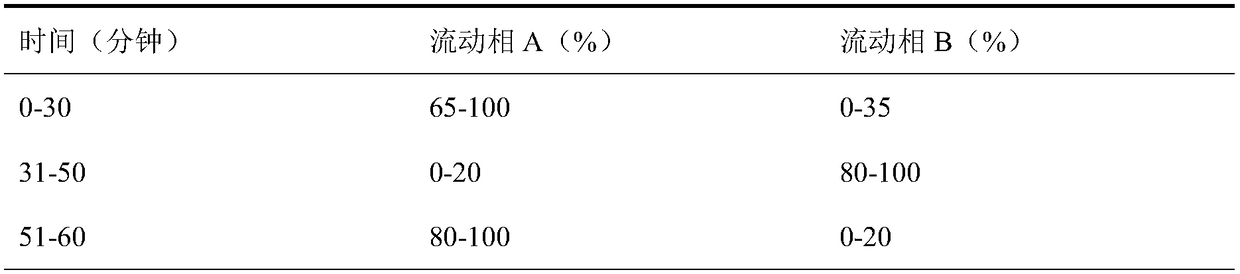
[0052] Mixed solution: accurately weigh ornithine aspartate, place it in a 10ml measuring flask, accurately measure 3-amino-2-piperidone, α-aspartic acid dimer, aspartic acid condensate, Place 0.5ml of the dimer arginine reference substance positioning solution in the same place, add mobile phase B to dis...
Embodiment 2
[0058] Example 2 Measurement of linear range
[0059] 1) Solution configuration
[0060] Precisely weigh 10mg each of α-aspartic acid dimer, aspartic acid condensate, and dimer arginine, put them in a 10ml volumetric flask, add an appropriate amount of solvent to dissolve and dilute to the mark, shake well, as impurities Linear stock solution.
[0061] Precisely weigh each 10mg of aspartic acid and ornithine, and place them in a 10ml measuring flask. Precisely add 2ml of the above-mentioned impurity linear stock solution and place in a 100ml measuring flask. Add an appropriate amount of solvent to dissolve and dilute to the mark. Shake well, as linear Test solution 1#.
[0062] Precisely measure linear test solution 1#8ml, 7ml, 6ml, 5ml, 4ml, 3ml, 2.5ml, 1ml, 0.5ml, put them into 10ml measuring flasks, add solvent to dilute to the mark, shake well, and use as linear test solution 2# , 3#, 4#, 5#, 6#, 7#, 8#, 9#, 10#.
[0063] Accurately weigh 40mg of 3-amino-2-piperidone into a 100ml...
Embodiment 3
[0088] Example 3 Determination of detection limit
[0089] 1), the method of detection limit determination
[0090] Configuration of detection limit solution: Dilute the linear stock solution of each impurity in Example 2 step by step, and obtain the solution when S / N≈3 as the detection limit solution;
[0091] 2), measurement results
[0092] Precisely take 20μl of the blank solution and inject it into the liquid chromatograph; Precisely take 20μl of the above-mentioned limit of quantification solution, inject it into the liquid chromatograph, and inject 3 consecutive samples, record the chromatogram, take S / N≈3 as the detection limit, and the statistics are as follows .
[0093] Table 8: Detection limit test results
[0094]
[0095] Test results: The detection limit of aspartic acid condensate was 0.6ng, the detection limit of α-aspartic acid dimer was 1.0ng, the detection limit of dimer arginine was 3.4ng, and the detection limit of ornithine was 16.7ng. The detection limit of aspa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com