Dutasteride soft capsule and preparation method thereof
A technology for dutasteride and soft capsules, applied in the field of improved dutasteride soft capsules and the preparation thereof, can solve the problems of slow disintegration, low dissolution of dutasteride soft capsules, unstable quality, etc. The effect of promoting disintegration, ensuring gastrointestinal safety, and avoiding safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0041] Embodiments 1-5 and Comparative Example: Preparation of Dutasteride Soft Capsules
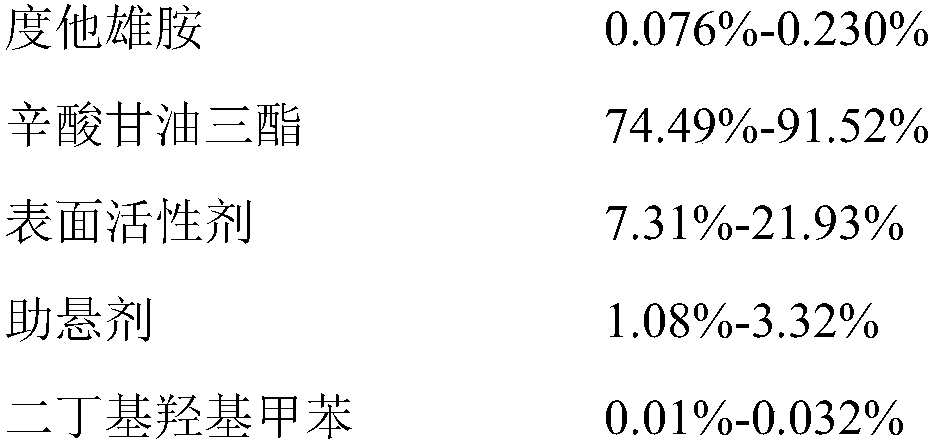
[0042] 1. Preparation of Example Samples: According to the prescription of dutasteride soft capsules of Examples 1 to 5 of the present invention in Table 2, 1000 dutasteride soft capsules were prepared according to the following steps:
[0043] (1) Preparation of contents: Weigh the prescribed amount of caprylic triglyceride and heat it to 55-65°C, add the prescribed amount of raw materials dutasteride, surfactant, suspending agent and dibutyl hydroxytoluene, and stir until Dissolve completely, and control the temperature at 50°C to 60°C;
[0044] (2) Preparation of soft capsule shell: Weigh the prescribed amount of glycerin, disintegrating agent, opacifier and purified water, heat to 75-80°C in a chemical glue tank, mix evenly with gelatin under stirring condition, and vacuum remove Air, filtered for use;
[0045] (3) Pill making: the contents containing dutasteride are divided into s...
Embodiment 6
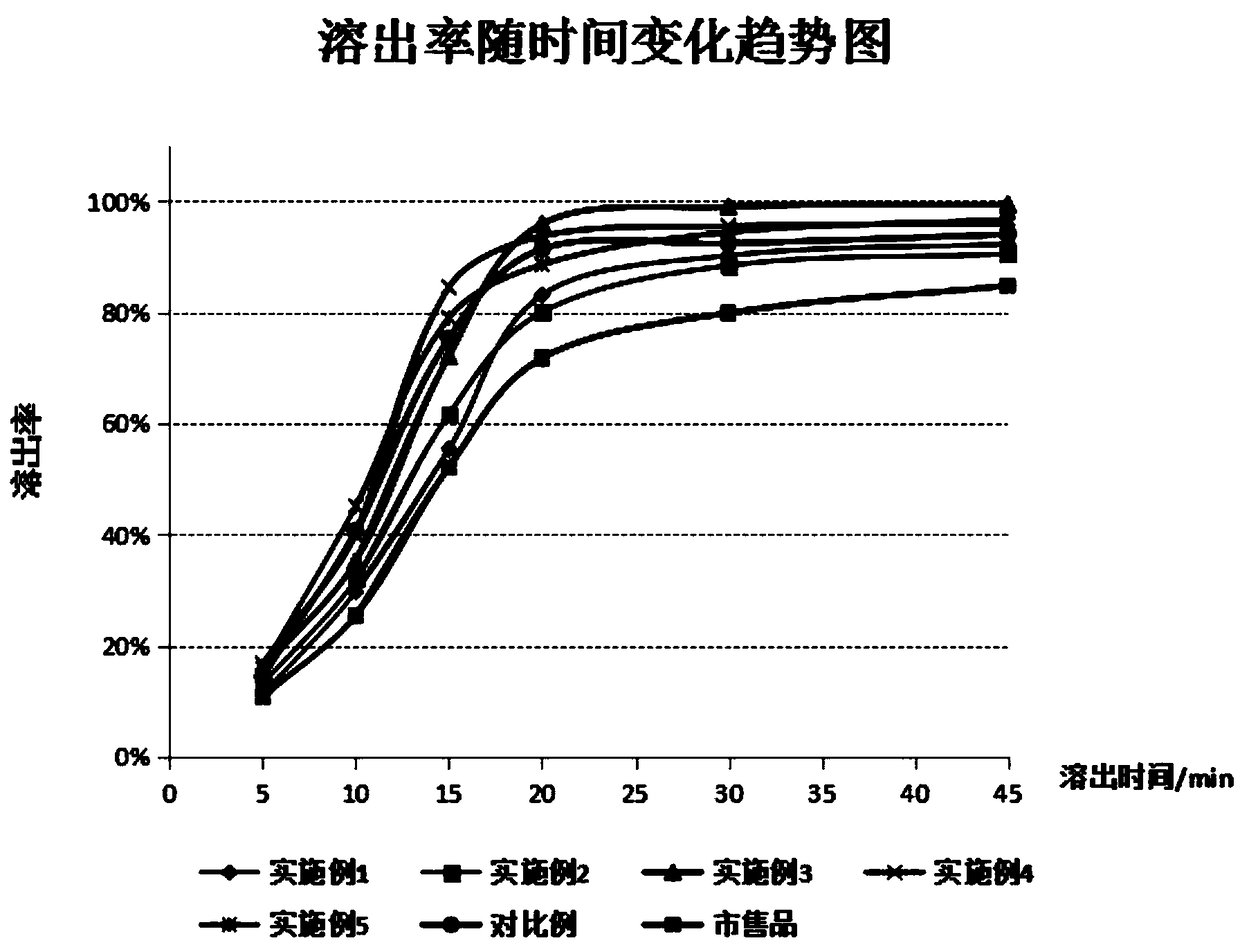
[0051] Embodiment 6: investigation on dissolution rate of dutasteride soft capsule
[0052] Sample preparation: prepare respective samples according to Examples 1-5 and comparative examples, and set aside. Commercially available dutasteride with a storage period similar to that of the prepared samples was purchased for investigation. The following other embodiments are the same.
[0053] Investigation method: according to the basket method in the "0391 Dissolution and Release Determination Method" in the 2015 edition of "Chinese Pharmacopoeia", the rotating speed is 50 revolutions / min, and the market conditions of the examples and samples, and dutasteride soft capsules are taken respectively. For commercial products, each 6 capsules were tested for dissolution rate. Measure the dissolution rate in 900ml 2.0% SDS 0.1mol / L hydrochloric acid solution (simulated gastric juice) at 37±0.5°C, take 5mL samples regularly at 5, 10, 15, 20, 30, and 45 minutes respectively, and use HPLC...
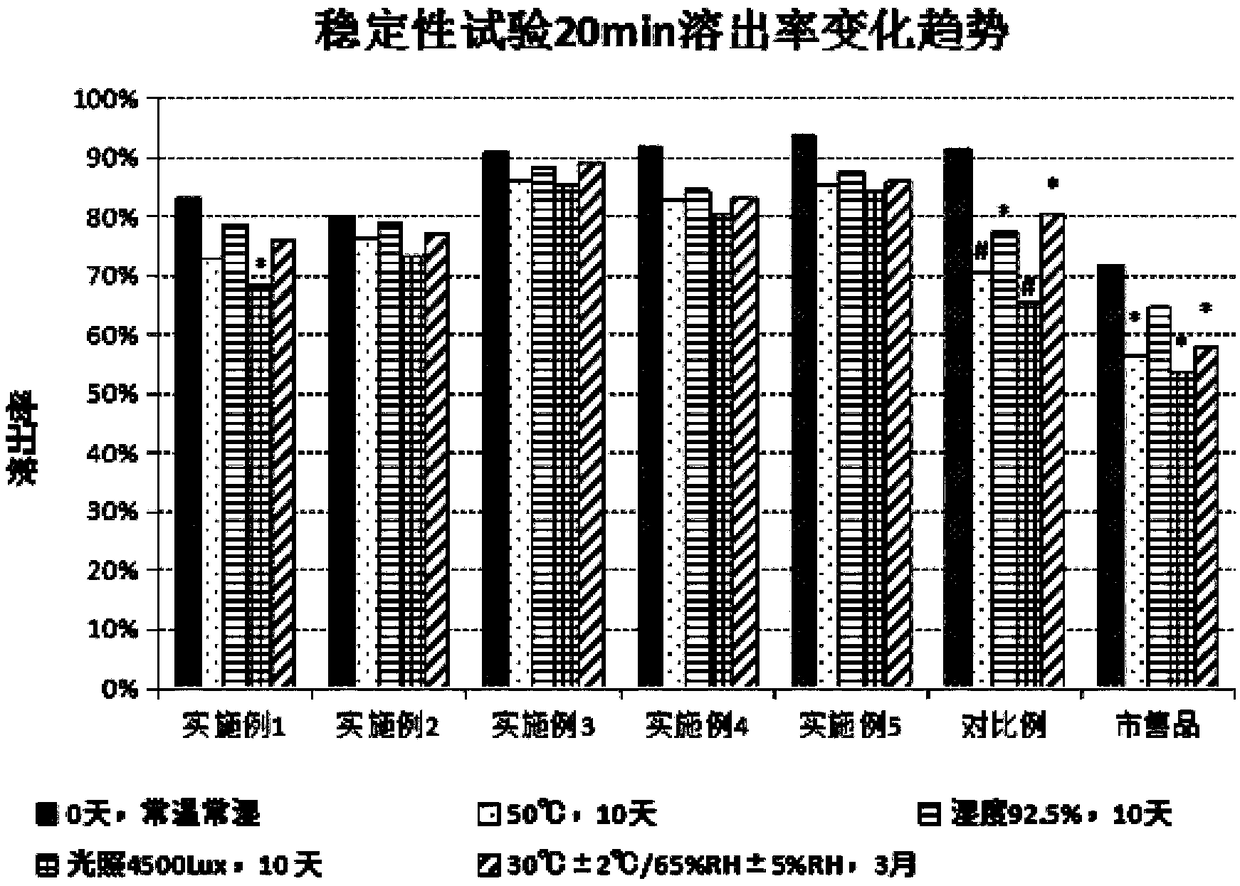
Embodiment 7
[0056] Example 7: Investigation of Dutasteride Soft Capsules Crosslinking Degree and Disintegration Time
[0057] Inspection method:
[0058] (1) Processing of the sample capsule shell: take the example sample, comparative example sample and commercially available product, cut the soft capsule sample along the middle slit, extrude the contents, wash the capsule shell 3 times with absolute ethanol, and dry to The water content is about 10%, and it is reserved.
[0059] (2) Determination of equilibrium swelling amount (Seq): different samples were placed at 40° C. and RH 75% for 3 months, and samples were taken regularly. For each sampling, take 6 pieces of sample films from different sample capsules, label them separately, weigh them precisely, place them in 500m1 distilled water at 20°C for swelling, take out the films at 1, 2, 2.5, 3, 3.5, and 4 hours and wipe off the surface with filter paper liquid, precisely weighed. In addition, 6 pieces were taken and dried at 105°C t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com