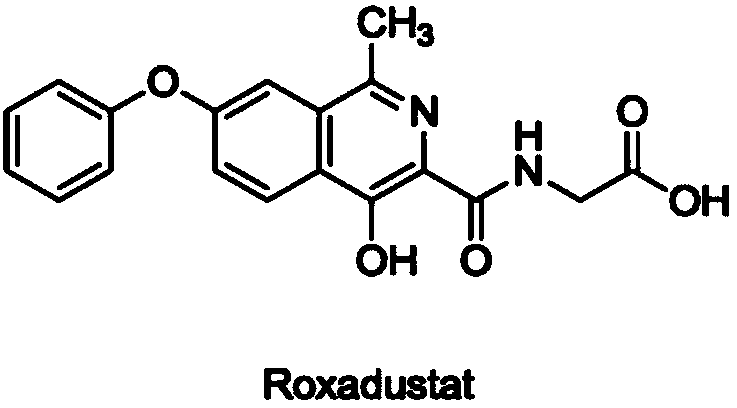
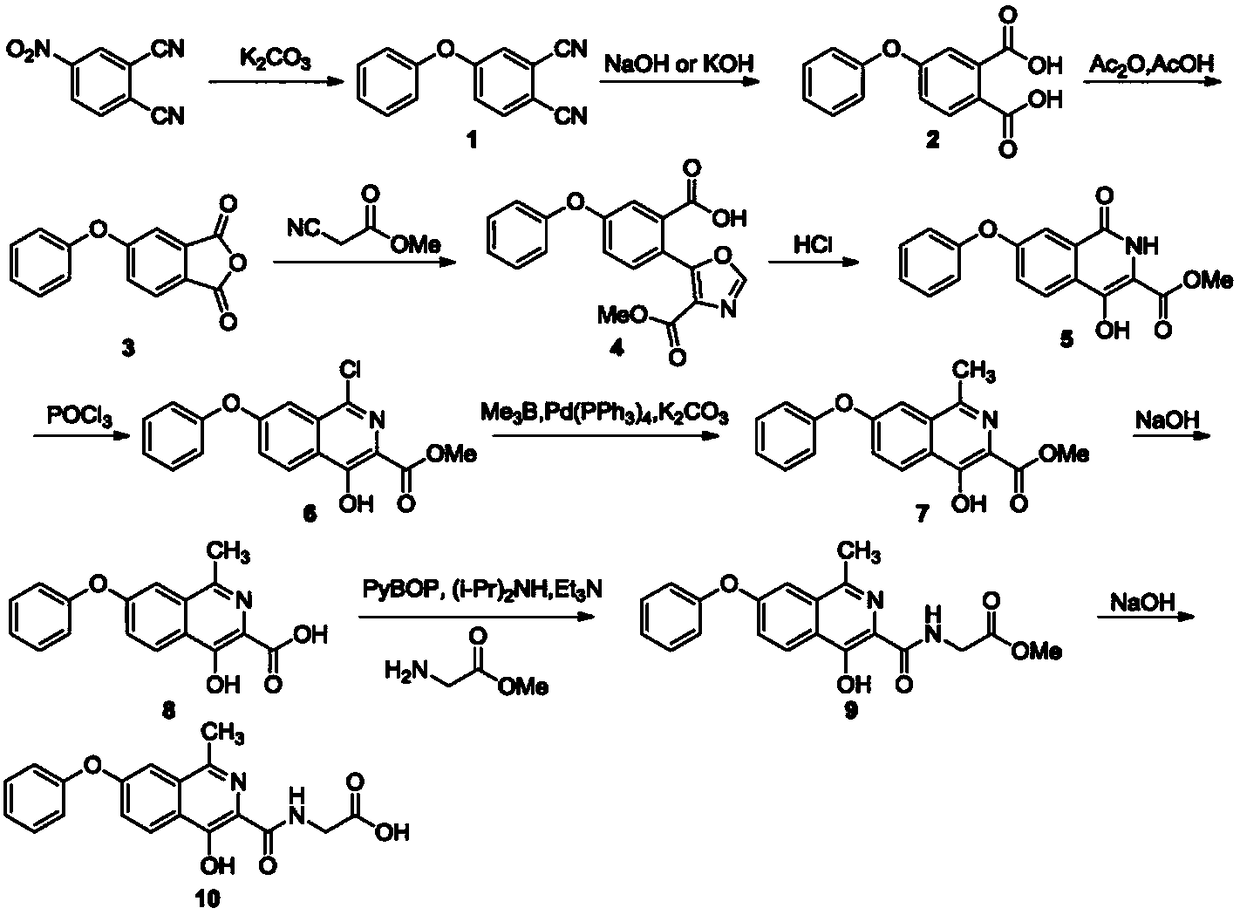
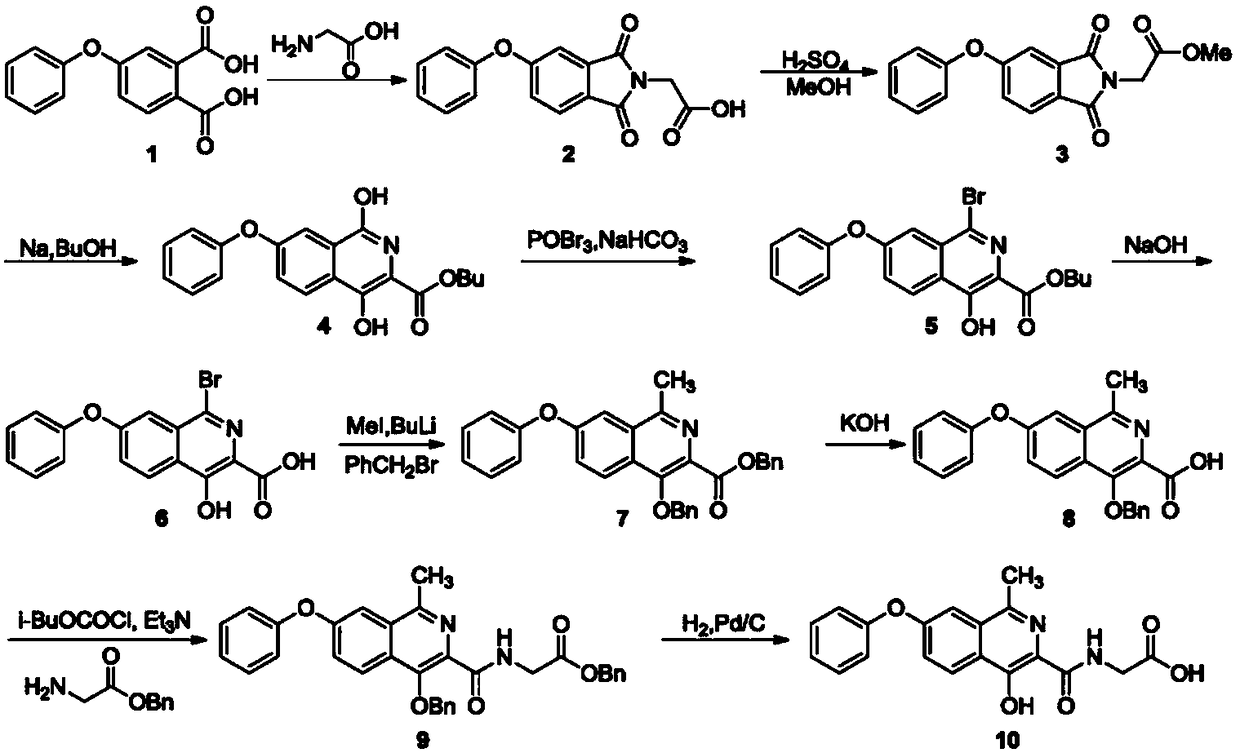
Synthesis method of roxadustat
A technology that can be used to generate compounds and compounds, applied in the field of medicine and chemical industry, can solve the problems of difficult industrial production, expensive starting materials, harsh reaction conditions, etc., and achieve the effects of short production cycle, low cost and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] The preparation of embodiment 1 compound B
[0101] Add 136.5ml of acetic acid and compound A (27.3g, 0.1mol) to the reaction flask, slowly add acetic anhydride (61.2g, 0.6mol) under stirring, and then heat up to 65--70°C for 2-3 hours. The solvent was distilled off under reduced pressure, 300 ml of 80% ethanol aqueous solution was added, stirred, filtered, and dried to obtain 35.3 g of solid compound B with a yield of 99.0% and a purity of 95.6%.
Embodiment 2
[0102] The preparation of embodiment 2 compound C
[0103] Add 357.0ml of anhydrous ethyl alcohol and compound B (35.7g) to the reaction flask, add concentrated sulfuric acid (1ml), raise the temperature to 70-78°C for 2-3 hours, after completion, evaporate the solvent under reduced pressure, add 500ml of water / 200ml of ethyl acetate After ester extraction and liquid separation, the organic layer was concentrated to dryness under reduced pressure to obtain 37.0 g of compound C with a yield of 96.2% and a purity of 92.9%.
[0104] MS:[M+1]=386.1
[0105] 1 H NMR(400MHz,DMSO)δ8.50(s,1H)7.03-7.55(m,10H),5.15(d,1H),4.15(q,2H),2.23(s,3H),1.92(s,3H ), 1.25(t,3H).
Embodiment 3
[0106] The preparation of embodiment 3 compound D
[0107] Add 154.0ml of acetonitrile and compound C (38.5g) to the reaction flask, add phosphorus oxychloride (76.5g), raise the temperature to 78-80°C for 2-3 hours, after completion, evaporate the solvent under reduced pressure, add 500ml of water / 200ml of acetic acid Ethyl ester extraction, liquid separation, and the organic phase was concentrated to dryness under reduced pressure to obtain 31.2 g of compound D with a yield of 85.0% and a purity of 97.7%.
[0108] MS:[M+1]=368.1
[0109] 1 H NMR(400MHz,DMSO)δ7.02-7.58(m,8H),6.55(d,1H),4.11(q,2H),2.34(s,3H),2.20(s,3H),1.22(t, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com